Question
Question: (a)- Discuss cis-trans isomerization with suitable example (organic compounds). (b)- Account for t...
(a)- Discuss cis-trans isomerization with suitable example (organic compounds).
(b)- Account for the reducing nature of formic acid
Solution
To answer this question we should know about isomerization. Isomerization is the phenomena when two compounds differ in properties but can be represented by the same formula.
The formic acid is the first member of the homologous series of the carboxylic acids.
Complete step by step solution: Let’s discuss the answer of the first part:
Cis-trans isomerization is a type of geometric isomerization. It is that type of stereoisomerism in which the molecular formula remains same but the relative arrangement of the atoms differ. It is found in properly substituted double bonded compounds.
In the case of alkenes of the type abC=Cab, where a and b are substituents, the cis-trans isomerization is present.
If the identical substituents are on the same side then it is called as cis isomer of the compound. If the identical substituents are present on the opposite side then it is called a trans isomer of the compound.
Let’s take example of but-2-ene
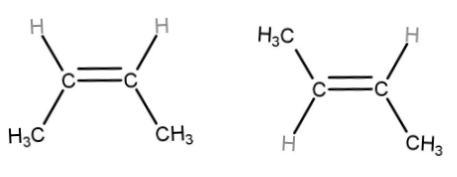
The first image is of cis-but-2-ene and the next image is of trans-but-2-ene.
Now, we will answer the part (b)
Formic acid is the only carboxylic acid which is a reducing agent. Formic acid is reducing in nature because of the aldehyde group attached to it. It is an atypical carboxylic acid and does not form anhydride. On heating in the presence of magnesium dioxide it further gets oxidized to carbon dioxide. This proves the reducing nature of formic acid.
Note: The cis and trans isomers are formed due to restricted rotation around a double bond. They differ in chemical and physical properties.
Formic acid is found in the ant sting and bee sting. The IUPAC name of formic acid is methanoic acid.
