Question
Question: A dipeptide 'z' on complete hydrolysis gives 'y' and 'z'. y on treatment with aq. $HNO_2$ produces l...
A dipeptide 'z' on complete hydrolysis gives 'y' and 'z'. y on treatment with aq. HNO2 produces lactic acid on the other hand 'z' on heating gives the following the given dipeptide is:
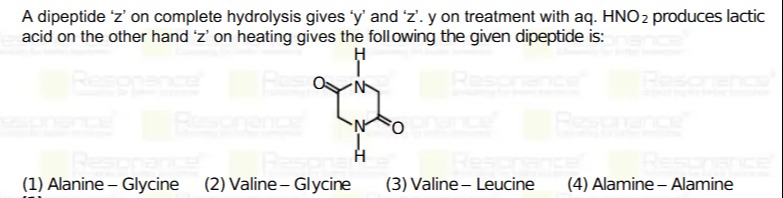
Alanine – Glycine
Valine – Glycine
Valine – Leucine
Alamine – Alamine
Alanine – Glycine
Solution
-
Hydrolysis: The dipeptide "z" when hydrolyzed gives two different amino acids: one is "y" and the other is "z".
-
Nitrous Acid Reaction: It is given that "y" on treatment with aqueous HNO2 gives lactic acid. Since lactic acid is derived on deamination of alanine (CH3CH(NH2)COOH→CH3CH(OH)COOH), "y" must be alanine.
-
Cyclization on Heating: The remaining amino acid "z" (which is not alanine) must be glycine because the dipeptide is unsymmetrical (giving two different products) and glycine (NH2CH2COOH) is known to undergo cyclization (by intramolecular nucleophilic attack resulting in a 2,5-diketopiperazine) more readily than more hindered amino acids.
-
Conclusion: Thus, the dipeptide "z" is made up of alanine and glycine.
