Question
Question: A diene, buta-1,3-diene was subjected to ozonolysis to prepare aldehydes. Which of the following ald...
A diene, buta-1,3-diene was subjected to ozonolysis to prepare aldehydes. Which of the following aldehydes will be obtained during the reaction?
A. CHO−CHO+2HCHO
B. CH3CHO+2HCHO
C. CH3CH2CHO+CH3CHO
D. 2CH3CH2CHO
Solution
The two step conversion of an alkene or alkyne into an ozonide followed by its reductive cleavage to yield carbonyl compounds is called ozonolysis. It occurs at a low temperature range of 196−200K.
Complete step by step answer:
Reaction description:
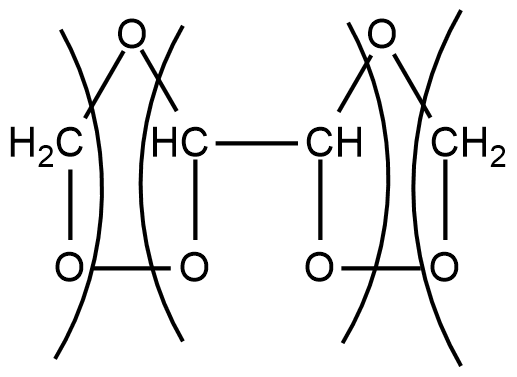
Step1: When ozone is passed through a solution of buta-1,3-diene in some inert solvent such as CH2Cl2,CHCl3 or CCl4 at low temperature of range 196−200K, it oxidises it into ozonides as given below:
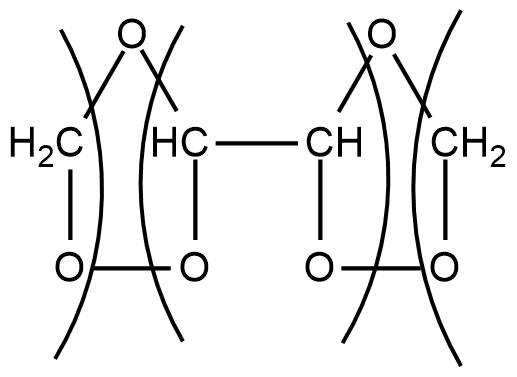
CH2=CH−CH=CH2+O3CH2Cl2196−200K
These ozonides are unstable compounds and also explosive in nature. So they are not isolated.
Step2: In second step, these ozonides are reduced, in situ, with zinc dust and water to give one molecule of CHO−CHO and 2 molecules of formaldehyde i.e. HCHO.
Zn/H2O−ZnOCHO−CHO+2HCHO
Hence the correct answer is option (A).
Ozonolysis has been exclusively used in the past for the structure elucidation of alkenes. It is the versatile method for locating the position of multiple bonds in an unknown alkene since no two alkenes give the same combination of aldehyde or ketones.
Note:
Ozonides are also reduced by H2/Pd . Sometimes ozonides give a mixture of ketone and aldehyde also; depending upon the structure of alkene. Instead of Zn/H2O or catalytic hydrogenation, ozonide can be more conveniently be reduced with dimethyl sulphide,(CH3)2S and it get oxidised itself to dimethyl sulphoxide.
