Question
Question: A diatomic ideal gas is heated at constant volume until the pressure is doubled and again heated at ...
A diatomic ideal gas is heated at constant volume until the pressure is doubled and again heated at constant pressure until the volume is doubled. Find the average molar heat capacity of the whole process.
A. 613R
B. 619R
C. 623R
D. 617R
Solution
The entire process consists of an isochoric process where the pressure increases and an isobaric process where the volume increases. Thus the heat absorbed for the entire process can be expressed as the sum of the heat absorbed in the isochoric process and the isobaric process. The ideal gas equation gives how the temperature changes for each process.
Formula used:
->The heat gained or lost in any thermodynamic process is given by, ΔQ=nCΔT where n is the number of moles present in the sample, C is the molar specific heat and ΔT is the change in temperature for the process.
->The ideal gas equation is given by, PV=nRT where P is the pressure of the sample, V is the volume of the sample, n is the number of moles present in the sample, R is the gas constant, T is the temperature of the sample.
Complete step-by-step solution:
Step 1: Sketch a P-V diagram for the whole process.
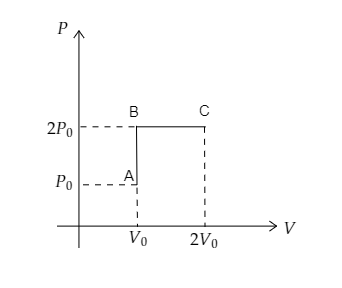
In the above diagram, the path AB refers to the isochoric process where the pressure is doubled and the path BC refers to the isobaric process where the volume is doubled.
We have taken the initial pressure of the gas at A to be P0 and the initial volume to be V0 .
At B the pressure of the gas will be P=2P0 while the volume remains the same as V0.
At C the volume doubles to become V=2V0 while the pressure remains to be P .
Step 2: Use the ideal gas equation to express the change in the temperature for the paths AB and BC.
The ideal gas equation is given by, PV=nRT --------- (1)
For the whole process, the number of moles n present in the sample remains constant.
From equation (1) we know that for the path AB the temperature change is proportional to the change in pressure as the volume remains constant along AB. The temperature doubles.
If TA=T0 is the initial pressure at A then at B it will be TB=2T0
⇒ΔTAB=TB−TA=2T0−T0=T0
For the path BC, as the pressure remains constant the temperature change will be proportional to the change in volume of the gas and so it doubles.
At B the temperature is TB=2T0 so at C it will be TC=4T0 .
⇒ΔTBC=TC−TB=4T0−2T0=2T0
Then the temperature change for the whole process ABC is given by, ΔTABC=TC−TA
Substituting for TC and TA in the above relation we get, ΔTABC=4T0−T0=3T0
Step 3: Express the relation for the heat absorbed in the isobaric and isochoric process.
The heat absorbed in the isochoric process is given by, ΔQAB=nCVΔTAB -------- (2)
where CV is the molar specific heat at constant volume.
Substituting for CV=25R and ΔTAB=T0 in equation (2) we get, ΔQAB=n25RT0
The heat absorbed in the isobaric process is given by, ΔQBC=nCPΔTBC -------- (3)
where CP is the molar specific heat at constant pressure.
Substituting for CP=27R and ΔTBC=2T0 in equation (3) we get, ΔQBC=n27R2T0
Thus the heat absorbed during the isochoric process is ΔQAB=n25RT0 and that for the isobaric process is ΔQBC=n27R2T0 .
Step 4: Express the heat absorbed for the whole process ABC to find the average molar specific heat.
The heat absorbed for the whole process ABC is given by, ΔQABC=nCΔTABC ------- (4)
The heat absorbed for the entire process can also be expressed as ΔQABC=ΔQAB+ΔQBC----- (5)
Combining equations (4) and (5) we get, nCΔTABC=ΔQAB+ΔQBC ------ (6)
Substituting for ΔTABC=3T0 , ΔQAB=n25RT0 and ΔQBC=n27R2T0 in equation (6) we get, nC3T0=n25RT0+n27R2T0
⇒3C=25R+14R
⇒C=619R
Thus the average molar specific heat capacity is obtained as C=619R .
So the correct option is B.
Note:- For an isochoric process, the volume of the system remains constant and for an isobaric process, the pressure remains constant. The ideal gas is said to be diatomic. For a diatomic gas having f=5 degrees of freedom, the molar specific heat at constant volume is given by, CV=2fR=25R while the molar specific heat at constant pressure is given by, CV=(1+2f)R=27R
