Question
Question: (a) Describe briefly three experimentally observed features in the phenomenon of photoelectric effec...
(a) Describe briefly three experimentally observed features in the phenomenon of photoelectric effect.
(b) Discuss briefly how wave theory of light cannot explain these features.
OR
(a) Write the important properties of photons which are used to establish Einstein’s photoelectric equation.
(b) Use this equation to explain the concept of (i) threshold frequency and (ii) stopping potential.
Solution
The photoelectric effect is observed on a metal surface, when a light beam of a certain frequency is incident the electrons on the surface of the metal are ejected. It explains the particle nature of light. This has resulted in the explanation of the wave-particle duality of light.
Formula used:
E=W0+K.E.
W0=hυ0
eVo=(K.E.)max
Complete answer:
Before, engaging in the experiment of the photoelectric effect, let’s learn how the photoelectric effect came to be known.
Initially, Newton put forth the ‘corpuscular theory of light’. It states that – light is made up of small and individual particles called corpuscles. But this could not explain the diffraction, polarization, and interference properties of light, while Huygens’s wave theory did. As a result, it was not accepted.
Later, Max Planck propounded that light is made up of tiny packets of energy called quanta or photons. Based on this statement, Einstein postulated the photo-electric effect.
In the photoelectric effect, when the light beam is concentrated on a metal surface the electrons are ejected. As the light contains particles called photons, these electrons that are ejected due to photons are known as photoelectrons.
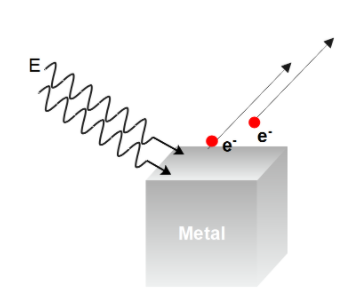
The equation of the photoelectric effect is given by
E=W0+K.E.
Where,
E is the energy of the incident photons
W0 is the work function
K.E. is the kinetic energy of the photoelectrons.
The important observations of the photoelectric effect were –
1. The photoelectric effect is instantaneous, i.e., the time lag between the incidence of the light beam and the emission of the photoelectron is too small or non-existent.
2. The number of photoelectrons that are emitted depends on the intensity of the incident light. But the energy of the emitted photoelectrons does not.
3. The energy of the emitted photons is directly proportional to the frequency of the incident light beam. i.e. written mathematically as
E∝υ⇒E=hυ
Here,
E is the energy of the incident beam
υ is the frequency of the incident beam
h is the Planck’s constant
The photoelectric effect laid a strong base for the particle nature of light. The wave theory of light fell short in explaining the photoelectric effect. The particle theory explained that the collisions that take place between the photons and the electrons on the metal surface were the reason for the emission of photoelectrons due to the exchange of energies.
Now, let’s understand some important properties of the photons –
1. Photons are tiny packets of energy, which make up light. They do not possess mass or an electric charge.
2. The energy carried by the photon varies according to the frequency of the light beam.
3. They travel at the speed of the light, in free space.
4. Their energy can be destroyed or created, as in the absorption and emission of radiation.
It was observed in the experiment that the light beam produced the photoelectrons only when the frequency of the light beam was more than a certain value, i.e. all light beams of different frequencies did not produce photoelectrons. The minimum frequency needed for the electrons to be ejected from the metal surface is known as the threshold frequency. Consider Einstein’s photoelectric equation E=W0+K.E. here the work function or the threshold energy is the minimum energy needed for the emission of photoelectrons. It is given by W0=hυ0. And the variable ‘υ0’ is the threshold frequency. Below the threshold frequency, no electrons are emitted from the surface of the metal. The threshold frequency varies from metal to metal.
The photoelectrons are subjected to a potential difference to direct the current towards a collector. The current obtained by the photoelectrons is called photo-electric current. Once the frequency and the intensity of light are fixed the photoelectric current increases with an increase in positive voltage. If the photo-electrons are subjected to an increasingly negative voltage, there is no emission of photoelectrons i.e., no photoelectric current is seen. This voltage is known as stopping potential or stopping voltage. It is given by
eVo=(K.E.)max
Here,
Vo is the stopping potential
(K.E.)max is the maximum kinetic energy
e is the charge of the electron
The above statement indicates the energy supplied by the stopping potential must be equal to the maximum kinetic energy, for the photoelectric current to be zero.
Note:
You must keep in mind that the kinetic energy of the photoelectron may vary depending on the incident energy. When the incident energy is given, it is first used in ejecting the electron, i.e. threshold energy. The remaining energy is then used by the ejected electron to move with a certain velocity.
