Question
Biology Question on Biomolecules
A dehydration reaction links two glucose molecules to produce maltose. If the formula for glucose is C6H12O6 then what is the formula for maltose?
C12H20O10
C12H24O12
C12H22O11
C12H24O11
C12H22O11
Solution
In a dehydration reaction, two molecules of the sugar glucose (monomers) combine to form a single molecule of the sugar maltose. Two glucose molecules should combine to form a disaccharide C12H24O12 . However, due to dehydration, one H2O molecule is eliminated and the final product is C12H22O11.
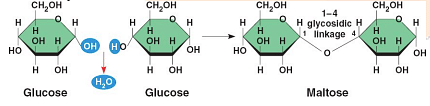
The formula for maltose is C12H22O11
Maltose is formed by a dehydration reaction between two glucose molecules. In each glucose molecule C6H12O6, one molecule of water (H2O) is eliminated. This results in the formation of a glycosidic bond between the two glucose units.
When two glucose molecules combine to form maltose, the formula for maltose can be determined by adding up the respective atoms from each glucose molecule while subtracting one water molecule:
(C6H12O6)+(C6H12O6)−H2O=C12H22O11
Therefore, the formula for maltose is C12H22O11
