Question
Question: A cylindrical container has a cross sectional area of $A_0 = 1cm^2$ at $0^\circ C$. A scale has been...
A cylindrical container has a cross sectional area of A0=1cm2 at 0∘C. A scale has been marked on vertical surface of the container which shows correct reading at 0∘C. A liquid is poured in the container. When the liquid and container is heated to 100∘C, the scale shows the height of the liquid as 83.33 cm. The coefficient of volume expansion for the liquid is γ=0.001∘C−1 and the coefficient of linear expansion of the material of cylindrical container is α=0.0005∘C−1. A beaker has 300cm3 of same liquid at 0∘C. The two liquids are mixed. Find the final temperature of the mixture assuming that heat exchange takes place between the liquids only, and its specific heat capacity is independent of temperature
31700∘C
Solution
The problem involves two main parts: first, determining the initial volume of the liquid in the container at 0∘C based on the scale reading at 100∘C and the thermal expansion properties of the liquid and the container; second, calculating the final temperature when this liquid is mixed with a known volume of the same liquid at a different temperature using the principle of calorimetry.
Part 1: Determine the initial volume of the liquid in the container at 0∘C.
Let A0 be the cross-sectional area of the container at 0∘C, and h0 be the actual height of the liquid column at 0∘C. The volume of the liquid at 0∘C is V0=A0h0. Given A0=1cm2. So, V0=h0.
When the liquid and container are heated to Tf=100∘C, the temperature change is ΔT=100∘C−0∘C=100∘C. The cross-sectional area of the container at 100∘C expands to Af. The coefficient of area expansion is βC=2αC, where αC is the coefficient of linear expansion of the container material. Af=A0(1+βCΔT)=A0(1+2αCΔT). Given αC=0.0005∘C−1. Af=1cm2(1+2×0.0005∘C−1×100∘C)=1(1+0.001×100)=1(1+0.1)=1.1cm2.
The volume of the liquid at 100∘C expands to Vf. The coefficient of volume expansion of the liquid is γL. Vf=V0(1+γLΔT). Given γL=0.001∘C−1. Vf=V0(1+0.001∘C−1×100∘C)=V0(1+0.1)=1.1V0.
At 100∘C, the liquid fills the container up to an actual height hf. The volume of the liquid at 100∘C is also Vf=Afhf. So, 1.1V0=1.1hf. Since V0=A0h0=1×h0=h0, we have 1.1h0=1.1hf, which implies hf=h0. The actual height of the liquid column remains the same at 100∘C as it was at 0∘C. This is because γL=2αC, which is the condition for the apparent expansion of the liquid level in a container with expanding area to be zero if the scale did not expand. However, the scale does expand.
The scale is marked at 0∘C. A length L0 at 0∘C becomes Lf=L0(1+αCΔT) at 100∘C. The scale shows a reading of hscale=83.33cm at 100∘C. This reading corresponds to a length h0′ at 0∘C such that the actual height hf is the expanded length of h0′. So, hf=h0′(1+αCΔT). The scale reading hscale is this length h0′. hscale=h0′=1+αCΔThf. Since hf=h0, we have hscale=1+αCΔTh0. Given hscale=83.33cm=3250cm. h0=hscale(1+αCΔT)=3250cm×(1+0.0005∘C−1×100∘C). h0=3250(1+0.05)=3250×1.05=3250×100105=300250×105=3025×105=65×105=25×35=2175=87.5cm. The actual height of the liquid at 0∘C is h0=87.5cm. The volume of the liquid in the container at 0∘C is V0,container=A0h0=1cm2×87.5cm=87.5cm3. This liquid is currently at 100∘C.
Part 2: Mixing of liquids.
We have two quantities of the same liquid:
- Liquid from the container: Initial temperature T1=100∘C. Volume at 0∘C is V0,1=87.5cm3.
- Liquid from the beaker: Initial temperature T2=0∘C. Volume at 0∘C is V0,2=300cm3.
Let ρ0 be the density of the liquid at 0∘C. The masses of the two quantities of liquid are m1=ρ0V0,1 and m2=ρ0V0,2. m1=ρ0×87.5. m2=ρ0×300.
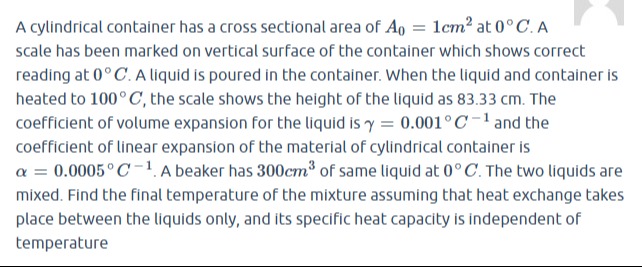
Let c be the specific heat capacity of the liquid, which is assumed to be independent of temperature. When the two liquids are mixed, assuming no heat exchange with the surroundings, the heat lost by the hotter liquid equals the heat gained by the colder liquid. Let Tf be the final temperature of the mixture. Heat lost by liquid 1 = m1c(T1−Tf). Heat gained by liquid 2 = m2c(Tf−T2). m1c(T1−Tf)=m2c(Tf−T2). (ρ0×87.5)c(100−Tf)=(ρ0×300)c(Tf−0). Assuming ρ0=0 and c=0, we can cancel them out. 87.5(100−Tf)=300Tf. 8750−87.5Tf=300Tf. 8750=300Tf+87.5Tf. 8750=387.5Tf. Tf=387.58750. 387.5=2775. Tf=27758750=7758750×2. 8750=875×10. 775=25×31. 875=25×35. Tf=25×31(25×35)×10×2=3135×10×2=31700. Tf=31700≈22.58∘C.
The final temperature of the mixture is 31700∘C.
Explanation of the solution:
- Calculate the actual height h0 of the liquid at 0∘C using the scale reading hscale at 100∘C and the thermal expansion of the container scale: h0=hscale(1+αCΔT).
- Calculate the volume of the liquid in the container at 0∘C: V0,container=A0h0. This liquid is at 100∘C when mixed.
- Identify the second liquid: 300cm3 at 0∘C. This is its volume at 0∘C, V0,beaker=300cm3.
- Use the principle of calorimetry: Heat lost by the hot liquid equals the heat gained by the cold liquid. m1c(T1−Tf)=m2c(Tf−T2).
- Express masses in terms of volumes at 0∘C and density at 0∘C: m=ρ0V0.
- Substitute values and solve for the final temperature Tf.
