Question
Question: A cycle followed by an engine (made of one mole of an ideal gas in a cylinder with a piston) is show...
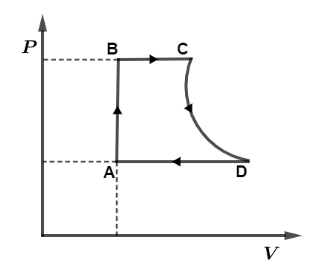
A cycle followed by an engine (made of one mole of an ideal gas in a cylinder with a piston) is shown in figure. The heat exchanged by the engine with the surroundings at constant volume is: Take (CV=23R)
A. (PB−PA)VA
B. 21(PB−PA)VA
C. 23[PBVB−PAVA]
D. 25[PBVB−PAVA]
Solution
In thermodynamics, we know that a cyclic process is one in which total internal energy is zero and work done is the area under P−V curve. Isobaric process is one in which pressure remains constant whereas an isochoric process is one in which volume remains constant. We will use these parameters on P−V to find the heat exchanged with the surrounding.
Formula used:
First law of thermodynamics can be written mathematically as dQ=dU+dW and dQ=CVdT where CV is known as specific heat at constant volume.
dW=PdV Is the work done by the gas.
Complete step by step answer:
From the given P−V curve we have, process AB is isochoric which is at constant volume so, dV=0 which means dW=0 then by using relation, dQ=dU+dW
dQAB=dUAB→(i)
Now, again we can use the relation of dQ=CVdT in order to find heat exchange in process AB
Q=TA∫TBCVdT
⇒Q=CV[TB−TA]
Now, it points of A and B use the ideal gas equation which is PV=nRT given that, n=1mole so
For point A we have, PAVA(R1)=TA
For point B we have, PBVB(R1)=TB put these values in equation Q=CV[TB−TA] we get,
Q=CV[PBVB−PAVA](R1)
Put (CV=23R) as given in the question
Q=23R[PBVB−PAVA](R1)
∴Q=23[PBVB−PAVA]
So, the heat exchanged by the gas with surrounding at constant volume is Q=23[PBVB−PAVA]
Hence, the correct option is C.
Note: It should be remembered that, an ideal gas is one which obeys the ideal gas equation perfectly which is written as PV=nRT where R is known as the universal gas constant and it’s a fixed constant having a value of 8.314JK−1mol−1 . Also work done in an isochoric process is always zero while in an isobaric process it’s written as Wisobaric=P(V2−V1) .
