Question
Question: A compound X with molecular formula \[{{\text{C}}_{\text{7}}}{{\text{H}}_{\text{8}}}\]is treated wit...
A compound X with molecular formula C7H8is treated with Cl2 in presence of FeCl3. Which of the following compounds are formed during the reaction?
A) 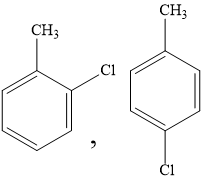
B) 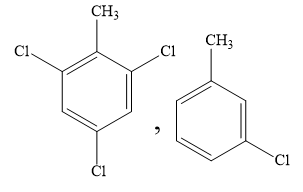
C) 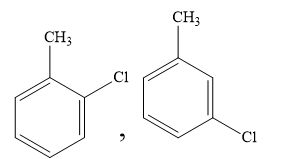
D) 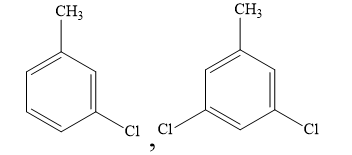
Solution
In the presence of lewis acid catalyst, aromatic compounds undergo electrophilic aromatic substitution reaction. The relative position of the incoming electrophile depends on the nature of the group already present in the ring. The group already present in the ring can be either ortho para directing group or meta directing group.
Complete step by step answer:
The compound X with molecular formula C7H8 is toluene. It is also called methyl benzene. It has one methyl group attached to a benzene ring.
Write the structural formula of toluene as shown below:
Toluene is an aromatic compound and undergoes electrophilic aromatic substitution reactions. You can replace one hydrogen atom of aromatic nucleus with suitable electrophile. You will need a Lewis acid catalyst to generate an electrophile.
Cl2 in presence of FeCl3 is the reagent used for the electrophilic aromatic substitution of aromatic compounds with chlorine. Here, FeCl3 is the Lewis acid catalyst. Thus, when you use Cl2 in presence of FeCl3 , you can replace one aromatic hydrogen atom of toluene with a chlorine atom to obtain an aryl chloride.
When one chlorine atom is present in the ring, the ring is deactivated to such an extent that di and tri substitution does not occur. Hence, you can rule out the options (B) and (D).
Toluene has a methyl substituent on benzene rings. This methyl substituent is an ortho para directing group in the electrophilic aromatic substitution reaction. So meta chlorotoluene is not formed in the reaction. So, you can rule out the option (C).
Hence, the products of the reaction will be ortho chloro toluene and para chloro toluene.
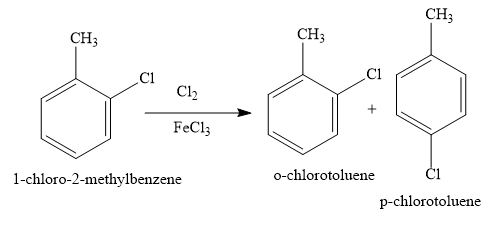
Hence, the correct option is the option (A).
Note: In an electrophilic aromatic substitution reaction, the methyl group is an activating group as it increases the electron density on the benzene ring through inductive effect and hyperconjugation. This increase in electron density is more at ortho and para positions than meta positions. Hence, methyl group is ortho para directing group.
