Question
Question: A compound X (having vinegar like smell) when treated with ethanol in the presence of the acid Z, gi...
A compound X (having vinegar like smell) when treated with ethanol in the presence of the acid Z, gives a compound Y which has a fruity smell, the reaction is:
C2H5OH+XZY+H2O
(i) Identify Y and Z
(ii) Write the structural formula of X
(iii) Name the reaction
Solution
First find out what is compound X. It says that it has a vinegar smell. So, the other name of vinegar gives you an idea what is X. Then, see what type of reaction it undergoes when the given reactant is treated with X to give water and a compound with fruity odour.
Complete step by step solution:
As per the given question,
Compound X is having a vinegar smell.
We know vinegar is also called acetic acid.
So, the compound X having vinegar smell is acetic acid, whose chemical formula is CH3COOH.
We can see that, in the given question the reactant given i.e. C2H5OHis an alcohol, having two carbon atoms and −OHas the functional group. This alcohol is called ethyl alcohol.
Now, let’s see which of the reaction processes produces water when an alcohol is treated with an acid.
So, we know that when an alcohol (ROH)[where R refers to a side chain or functional group] is treated with an acid (R′COOH) it undergoes esterification forming an ester (RCOOR′) and water. Esterification occurs in the presence of sulphuric acid (H2SO4) that acts as a catalyst.
Thus, now we got the compound X and Z that are acetic acid and sulphuric acid respectively.
And here, let’s consider R as the C2H5 of ethyl alcohol and R′as CH3of acetic acid. So, after esterification, the product formed as explained above will be C2H5−COO−CH3.
Looking forward to the chemical reaction that undergoes during esterification of the reactants we have, the following reaction is seen,
C2H5OH+CH3COOHH2SO4C2H5−COO−CH3+H2O
Thus, the product formed is ethyl acetate with chemical formula C2H5COOCH3 which will have a fruity smell.
So,
(i) The compound Y is ethyl acetate and Z is sulphuric acid.
(ii) Structural formula of X is,
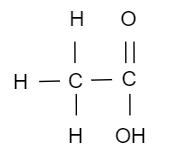
(iii) The reaction is called esterification.
Note: When a carboxylic acid is treated with an alcohol, esterification occurs in the presence of sulphuric acid (acts as a catalyst) which further forms ester and water. The ester formed generally has a pleasant characteristic i.e. fruity smell.
