Question
Question: A compound 'X' evolves CO₂ when treated with NaHCO₃ but gives negative test with tollen's reagent an...
A compound 'X' evolves CO₂ when treated with NaHCO₃ but gives negative test with tollen's reagent and fehling's solution. On treatment with CHCI₃, KOH, a foul smelling organic compound is formed. If 'X' do not give positive iodoform test, which of the following satisfy the conditions to be 'X'.
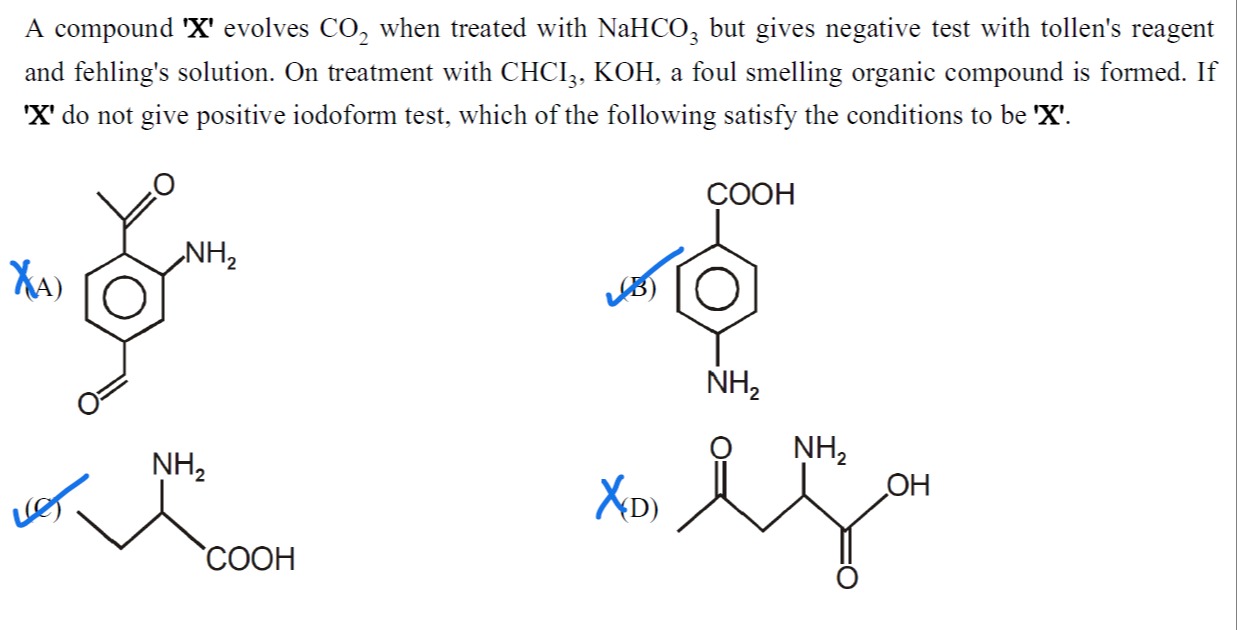
2-amino-4-acetylbenzaldehyde
4-aminobenzoic acid
Alanine (2-aminopropanoic acid)
4-amino-3-oxopentanoic acid
4-aminobenzoic acid and Alanine (2-aminopropanoic acid)
Solution
The compound 'X' must satisfy the following conditions:
- Evolves CO₂ when treated with NaHCO₃: This indicates the presence of a carboxylic acid group (-COOH).
- Gives negative test with Tollen's reagent and Fehling's solution: This indicates the absence of an aldehyde group (-CHO) and alpha-hydroxy ketone group.
- On treatment with CHCl₃, KOH, a foul smelling organic compound is formed: This indicates the presence of a primary amine group (-NH₂). This is the carbylamine reaction.
- Do not give positive iodoform test: This indicates the absence of a methyl ketone group (CH3CO-) and a methyl carbinol group (CH3CH(OH)-).
Let's examine each option:
-
Option A: 2-amino-4-acetylbenzaldehyde. Contains -CHO (aldehyde), -COCH₃ (methyl ketone), and -NH₂ (primary amine). It does not contain -COOH.
- Reacts with Tollen's/Fehling's (due to aldehyde).
- Gives positive iodoform test (due to methyl ketone).
- Gives positive carbylamine test (due to primary amine).
- Does not evolve CO₂ with NaHCO₃.
This option does not satisfy the conditions.
-
Option B: 4-aminobenzoic acid. Contains -COOH and -NH₂ (primary amine). It is an aromatic compound.
- Evolves CO₂ with NaHCO₃ (due to -COOH).
- Gives negative Tollen's/Fehling's test (no aldehyde or alpha-hydroxy ketone).
- Gives positive carbylamine test (due to primary amine).
- Gives negative iodoform test (no methyl ketone or methyl carbinol).
This option satisfies all the conditions.
-
Option C: Alanine (2-aminopropanoic acid). Contains -COOH and -NH₂ (primary amine). It is an aliphatic compound. The structure is CH3CH(NH2)COOH.
- Evolves CO₂ with NaHCO₃ (due to -COOH).
- Gives negative Tollen's/Fehling's test (no aldehyde or alpha-hydroxy ketone).
- Gives positive carbylamine test (due to primary amine).
- Gives negative iodoform test (no methyl ketone or methyl carbinol).
This option satisfies all the conditions.
-
Option D: 4-amino-3-oxopentanoic acid. Contains -COOH, -NH₂ (primary amine), and -COCH₂- (ketone, specifically a methyl ketone CH3CO-). The structure is CH3COCH2CH(NH2)COOH.
- Evolves CO₂ with NaHCO₃ (due to -COOH).
- Gives negative Tollen's/Fehling's test (ketones generally don't give these tests unless they are alpha-hydroxy ketones, which this is not).
- Gives positive carbylamine test (due to primary amine).
- Gives positive iodoform test (due to methyl ketone CH3CO-).
This option does not satisfy all the conditions.
Both Option B and Option C satisfy all the given conditions. Since the image shows ticks on both B and C, it is likely a multiple-choice question with multiple correct answers.
