Question
Question: A compound that gives a positive iodoform test is: \[ A.\;\;\;\;\;2 - \;pentanone \\\ B.\;...
A compound that gives a positive iodoform test is:
A.2−pentanone B.3−pentanone C.3−pentanol D.1−pentanolSolution
Hint : We must know that a compound that contains R−CO−CH3or R−CH(OH)−CH3can only give a positive iodoform test.
Complete step by step solution :
We must remember that the iodoform test is carried out to determine the presence of aldehyde (−CHO) and ketone (−C=O) having CH3−CO group. Positive iodoform test is given by compounds containing methyl keto group which is CH3−CO group.
Following are the compounds that give positive iodoform test:
Acetaldehyde (H−C=O)
Methyl Ketones (−C=O)
Ethanol (−C−OH)
Secondary Alcohols that contain Methyl Groups in Alpha Position
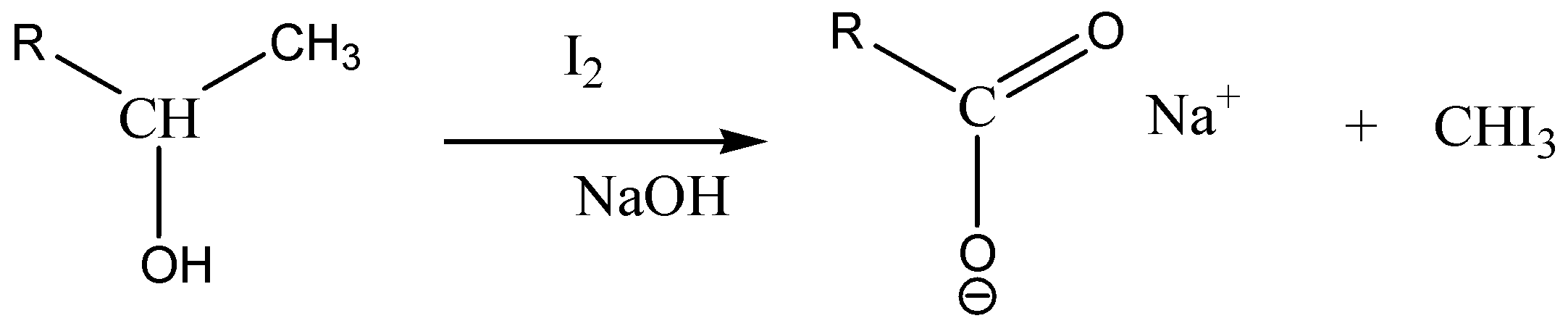
Iodoform test identifies the presence of carbonyl compounds with the structure R−CO−CH3or alcohols with the structureR−CH (OH)−CH3. In the iodoform test, iodine solution, a base (NaOHorKOH) and a methyl ketone reacts in such a way that gives a yellow precipitate along with a characteristic antiseptic smell.
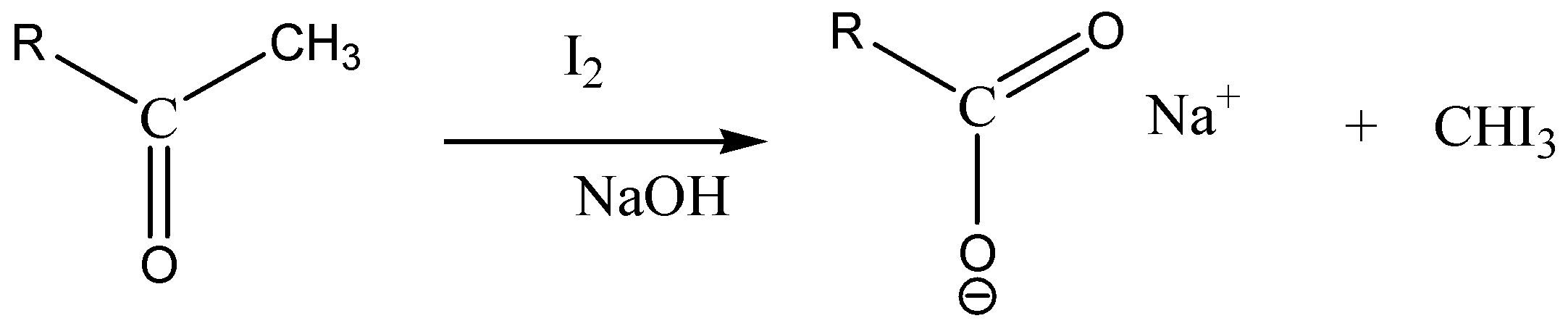
Following is a structure of 2−pentanone
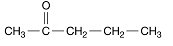
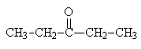
Following is a structure of3−pentanone
Following is a structure of3−pentanol. It is a secondary alcohol where pentane has a substitution at position 3 by a hydroxyl group.
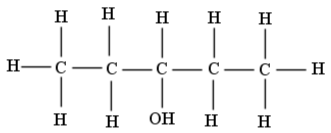
Following is a structure of 1−pentanol
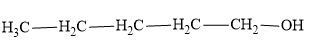
As we can see in the above chemical structures, among all options, 2−pentanoneis the only compound containing the CH3COgroup.
Hence, we can conclude that the option B is correct.
Additional information:
Positive iodoform test forms the pale yellow precipitate of iodoform which can be easily identified by its characteristic “antiseptic” smell.
Note : We must know that not all the alcohols give an iodoform test, only Secondary Alcohols containing Methyl Groups at Alpha Position will give a positive iodoform test.
