Question
Question: A compound contains \(28\) percent of nitrogen and \(72\) percent of a metal by weight. Three atoms ...
A compound contains 28 percent of nitrogen and 72 percent of a metal by weight. Three atoms of the metal combine with two atoms of N . Find the atomic weight of the metal :
24g
12g
72g
42g
Solution
Given that 28 of N and 72 of Metal in total its 3 atoms where Nitrogen are 2 atoms from 3. Finding the Equivalent weight of compound and then applying with given of both metal and then Equivalent weight with Valency will give the atomic weight of Metal.
Complete step by step answer:
According to problem, three atoms of M combine with 2 atoms of N
Thus, the formula of compound =M3N2
Equivalent Weight of N=314 ( Valency of N in compound is 3)
∴28g N combines with =72g metal
∴314 N combines with =2872×314=12g metal
∴ Equation weight of metal =12g
Atomic weight of metal = Equivalent weight × Valency
=12×2
=24[Valency]
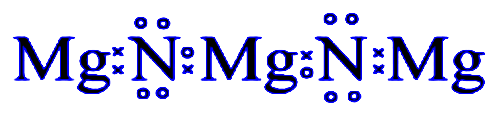
Additional Information: The metal is magnesium as its getting confirmed by its property and the compound in question is magnesium nitride M3N2
Note: Remember we have to keep this rule before solving, Mg has 2 valence electrons and loses these 2 valence electrons to form Mg2+ion. Similarly, N has 5 valence electrons. It gains 3 valence electrons to form N3− ion. Alternatively to balance charges, 3Mg2+ ion combines with two N3− ions to form M3N2.
