Question
Question: (a) Compound (A) with molecular formula \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}\text{O}\] g...
(a) Compound (A) with molecular formula C6H6O gives a violet color with neutralFeCl3. (A) Reacts with CHCl3 and NaOH gives two isomers (B) and (C) with the molecular formula C7H6O2. Compound (A) reacts with ammonia at 473K in the presence of ZnC12 gives compound (D) with the molecular formulaC6H7N. Compound (D) undergoes a carbylamine test. Identify (A), (B), (C), and (D) and explain the reactions.
(b) (A) is reddish-brown metal. It belongs to group 11 and period 4 of the periodic table. When heated below 1370K. (A) Given a black compound (B). When heated 1370K (A) gives a red compound (C). With concentrated nitric acid, (A) liberates NO2 gas and gives compound (D). Identify (A), (B), (C) and (D). Explain the reactions.
Solution
The conversion of reactant to the product depending on the reaction conditions mentioned on the arrow. Each reactant forms different products under the effect of reagents. Thus name reactions are the reactions that are carried out to get the determined product under the progress of the reaction.
Complete step by step answer:
a) Let’s find out the A, B, C, and D for the molecular formula C6H6O compound.
-First, we need to find compound A. According to the data given, the molecular formula C6H6O reacts with the neutral FeCl3to give a violet color.
-We know that a compound with a phenolic group reacts with the aqueous FeCl3 solution to give a violet, purple, blue color solution. The general reaction as shown below:
3C6H5OH+FeCl3→Fe(OC6H5)3+3HCl
Thus the given compound A is the phenol ,structure of it is as shown below,
Structure of compound A: C6H6O
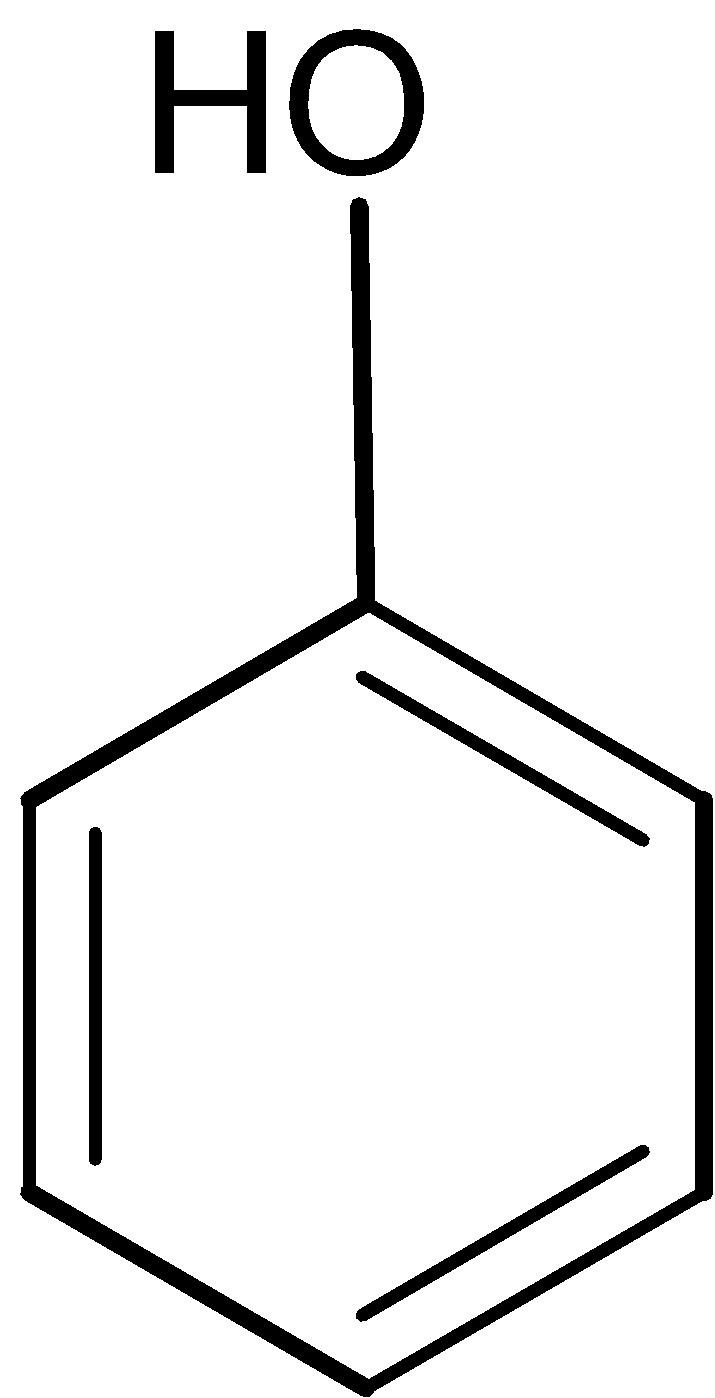
A=Phenol
-The compound A which is phenol reacts with the chloroform (CHCl3) a presence of NaOH to give phenol substituted with an aldehyde at ortho and para position to the benzene ring is called the Riemer-Tiemann reaction. The isomers have the formula C7H6O2. The ortho-substituted phenol is the major product of the reaction. The reaction of phenol to give o-hydroxybenzaldehyde and p-hydroxybenzaldehyde is as shown below:
Reaction:
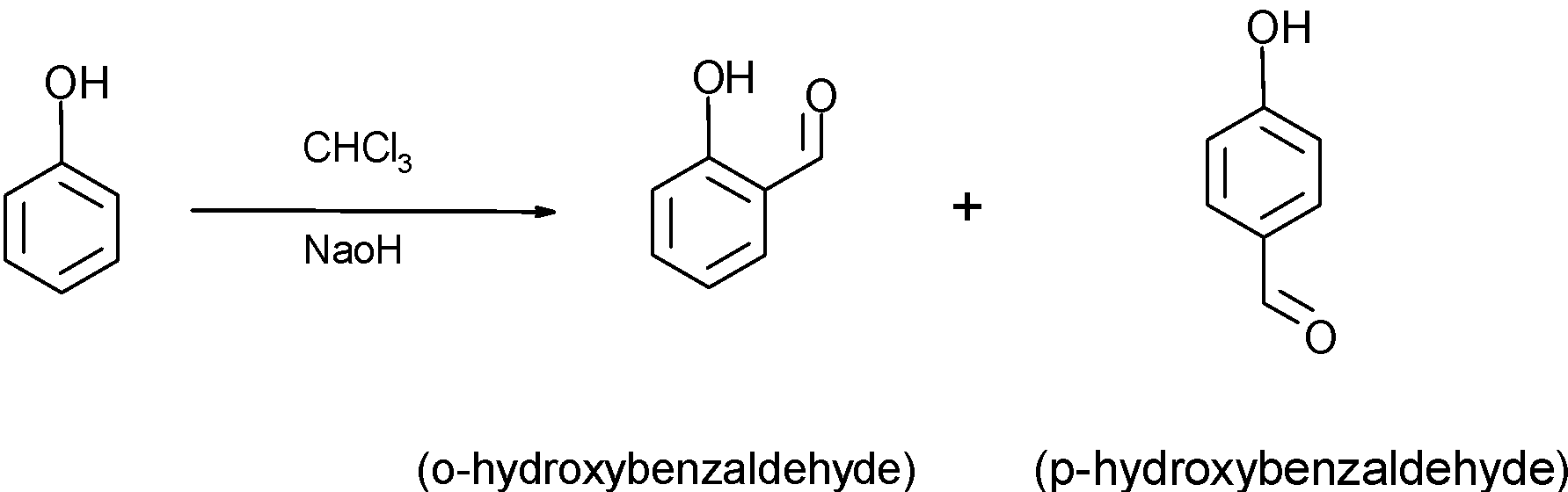
-when phenol reacts with the ammonia in presence of ZnCl2 at the temperature 47K, the -OH group of phenol reacts within NH3 and comes out as a water molecule, such that -NH2 the group attaches to the benzene ring to give the compound D as shown below,
Reaction:
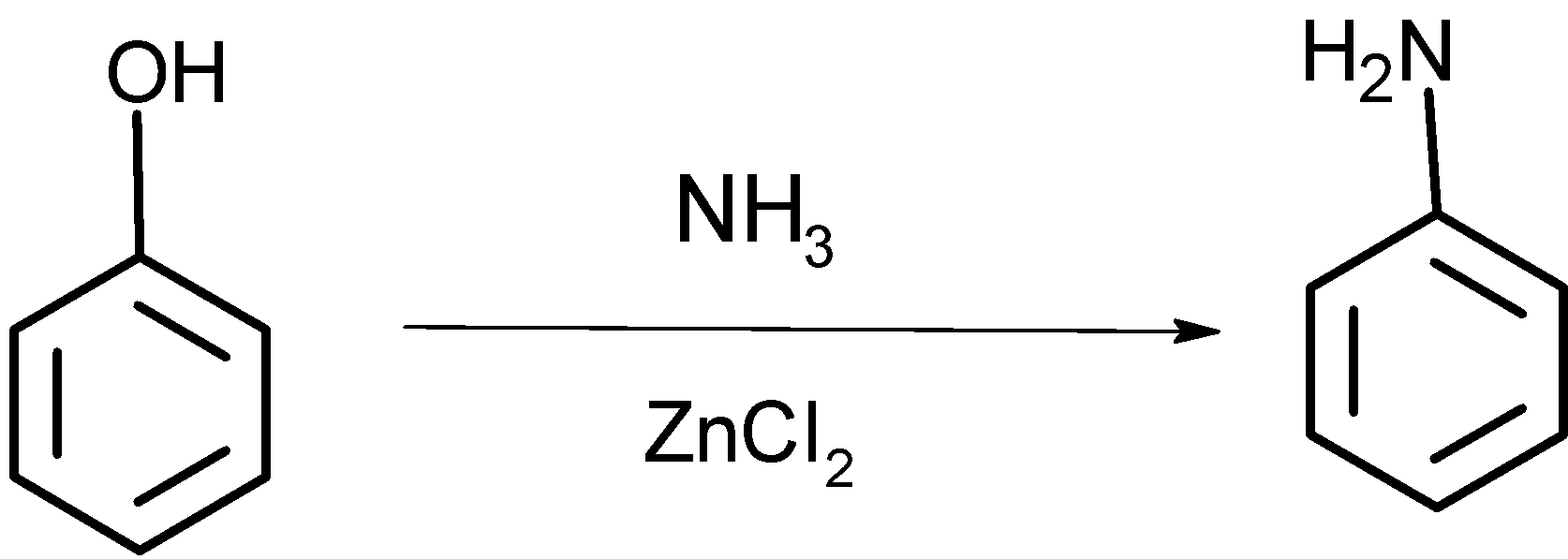
The compound D formed by the treatment of ammonia and zinc chloride undergoes a carbylamine test. As below:
Reaction:
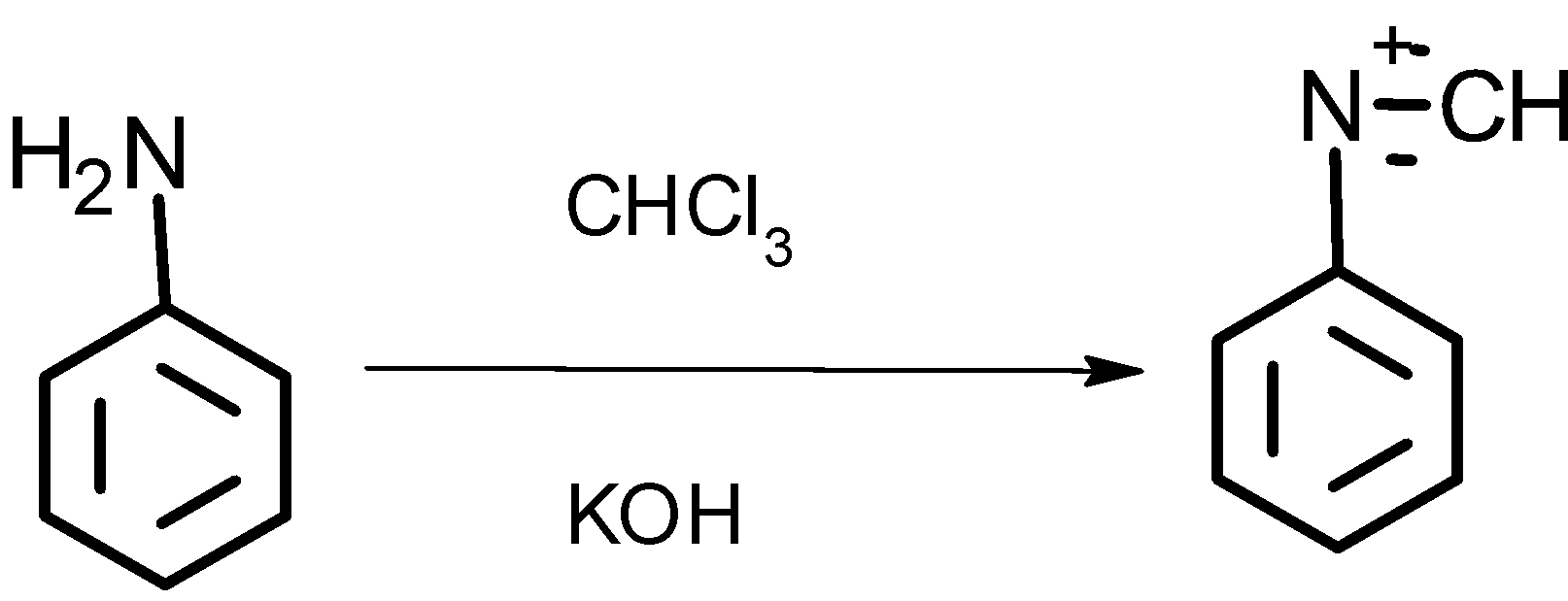
Thus compound D is a primary amine and its name is aniline
b) We are interested to find out the A, B, C, and D
-A is said to be the reddish-brown metal which belongs to group 11 and period 4 of the periodic table. If we search for the element in the periodic table, it is in the 3d transition metal series and group 11, the metal A is Copper (Cu).
-Copper is the reddish-brown metal that is lustrous. Its atomic number is 29 and it belongs to the 3d transition metal series.it is inorganic metal.
-Let's find out B,
When copper is heated to the 1370K it turns into the black. This is because when it is heated it reacts with the oxygen to form the black copper oxide. The reaction is as follows,
2Cu+O2→2CuO
CuO Is the black compound. Thus B is CuO.
-Let's find out the C,
When the copper is heated to the 1370K it turns into the black. This is because when it is heated it reacts with the oxygen to form the red copper (I) oxide or cuprous oxide .the reaction is as follows
4Cu+O2→2Cu2O
It is the oxide of copper when an excess of copper is heated in the presence of oxygen, thus cuprous oxide Cu2O is compound C.
-When the copper is treated with concentrated nitric acid, the copper nitrate is formed along with the liberation of nitro NO2 gas. The reaction is as follows,
Cu+HNO3→Cu(NO3)2+2NO2+2H2O
Thus, compound D is copper nitrateCu(NO3)2.
Note: These are concept based questions. The reagents play a very important role in the progress of the reaction. Thus to carry out the transformation of reactant to product always remember what is written on the arrow. For example, phenol undergoes the reaction in the presence of chloroform and alkali to give hydroxybenzaldehyde as the product. Try to figure out the line out for the mechanistic approach of reagent.
