Question
Question: (a) Compound (A) with molecular formula \({C_2}{H_4}O\) reduces Tollen’s reagent. (A) on treatment w...
(a) Compound (A) with molecular formula C2H4O reduces Tollen’s reagent. (A) on treatment with HCN gives compound (B). Compound(B) on hydrolysis with an acid gives compound (C) with molecular formula C3H6O3 which is an optically active compound. Compound (A) on reduction with N2H4/C2H5ONa gives a hydrocarbon (D) of molecular formula C2H6. Identify (A), (B), (C) and (D) and explain the reactions.
(b) Ionic conductance at infinite dilution of Al3+ and S042− are 189ohm−1cm2gmequiv−1 and 160ohm−1cm2gmequiv−1.Calculate equivalent and molar conductance of electrolyte at infinite dilution.
Solution
-The reaction with Tollen’s reagent is given by aldehydes and alpha-hydroxy ketones only. The reaction with HCN gives cyanohydrins which hydrolyse in the presence of an acid to give carboxylic acids and if any carboxylic acid has a chiral carbon, it is an optically active compound.
-According to Kohlrausch’s law, the sum of conductances of all ions is equal to the conductance of an electrolyte. Molecular formula gives the number of ions that will be formed.
Complete Step by step answer:
Since the Tollen’s reagent (Ammoniacal silver ) is reduced by aldehydes or alpha-hydroxy ketones, the compound (A), having the formula C2H4O can only be an aldehyde and not alpha-hydroxy ketone as it contains only one oxygen atom. So, the structural formula of compound (A) will be that of an acetaldehyde or ethanal.
Compound (A) is acetaldehyde or CH3CHO
The reaction of acetaldehyde with HCN will be a nucleophilic addition reaction which will give cyanohydrin. It is depicted below:
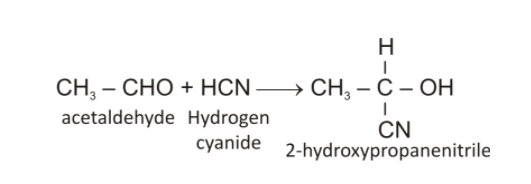
So, compound (B) is 2-hydroxypropanenitrile
Hydrolysis of nitriles gives carboxylic acid in which theCN group is changed to COOH group. Therefore, Compound (C) would be 2-hydroxypropanoic acid or commonly known as lactic acid.
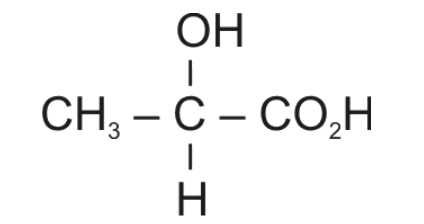
It is an optically active compound because alpha-carbon or the carbon directly attached to -COOH group is chiral carbon as it is attached to four different groups.
Compound (D) which is got by reduction of compound (A) by a strong reducing agent, N2H4 or hydrazine, is ethane having the formula C2H6
Therefore, compound (A) is acetaldehyde, Compound (B) is 2-hydroxypropanenitrile, compound (C) is lactic acid, and compound (D) is ethane.
Given:
Ionic conductance of Al3+ at infinite dilution:
λeq0(Al3+)=189ohm−1cm2(g−equiv)−1
Ionic conductance of S042− at infinite dilution:
λeq0(SO42−)=160ohm−1cm2(g−equiv)−1
Finding the equivalent conductance at infinite dilution
Now, the electrolyte in question is Al2(SO4)3which contains two Al3+ ions and three S042−ions
Therefore,
Λeq0(Al2(SO4)3=2×λeq0(Al3+)+3×λeq0(SO42−)
= > {{\Lambda }}_{eq}^0\left( {A{l_2}{{\left( {S{O_4}} \right)}_3}} \right) = 2 \times 189 + 3 \times 160$$$$ = 858\;oh{m^{ - 1}}c{m^2}{\left( {g - equiv} \right)^{ - 1}}
This is the equivalent conductance of an electrolyte at infinite dilution.
Converting the equivalent conductance obtained in step 1 to molar conductance at infinite dilution
Λm0=858ohmcm2×(g−equiv)1×1mol6(g−equiv)=5148ohm−1cm2mol−1
1 mole of Al2(SO4)3is equal to 6 g-equivalents as the total positive or negative valency is 6 in this particular electrolyte.
Note: - Remember that Tollen’s reagent is a weak oxidizing agent and it is reduced by only those carbonyl compounds in carbonyl group are not surrounded by alkyl groups, i.e. aldehydes or alpha-hydroxy ketones. Further, HCN will undergo breakage to give CN−1 which is a good nucleophile and will attack carbonyl carbon of aldehyde and since that carbon is unsaturated, it will be addition reaction. Further, hydrazine will reduce aldehyde into its corresponding alkane and not alcohol as it is a very strong reducing agent.
-The number of ions must be taken into account while adding the ionic conductances to get the overall electrolyte conductance. For this, the formula of an electrolyte must be deduced through valency exchange or criss-cross method. Molar conductance can be derived from equivalent conductance by just changing the units, i.e. (g-equiv) to mol. The knowledge that molar mass needs to be divided by total positive or total negative valency to get equivalent mass in case of ionic salts helps in conversion.
