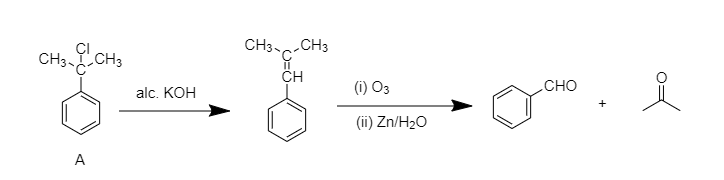
Question
Question: A compound ‘A’ with molecular formula \({ C }_{ 10 }{ H }_{ 13 }{ Cl }\) gives a white precipitate o...
A compound ‘A’ with molecular formula C10H13Cl gives a white precipitate on adding silver nitrate solution. ‘A’ on reacting with alcoholic KOH gives compound ‘B’ as the main product. B on ozonolysis gives C and D. C gives Cannizaro’s reaction but not aldol condensation. D gives aldol condensation,but not Cannizaro reaction A is:
(A) 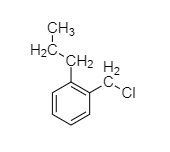
(B) 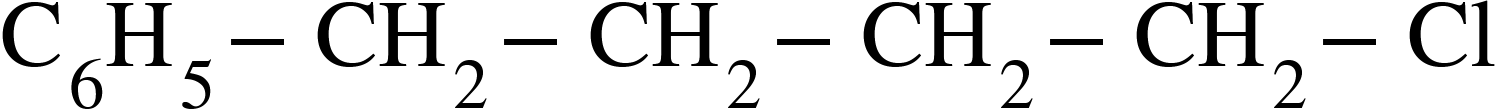
(C) 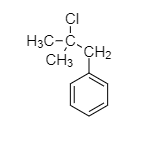
(D) 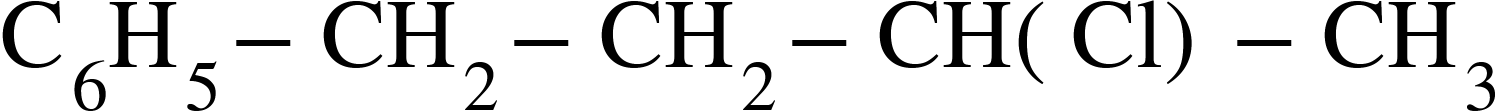
Solution
Ozonolysis involves the process of the addition of ozone to an alkene or alkyne resulting in cleavage of the double bond. Simply, the process of treating an organic compound with ozone to form an ozonide. used to locate double bonds in molecules.
Complete step by step solution:
Aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxy aldehyde or β-hydroxy ketone, followed by a dehydration to give a conjugated enone. Aldol condensations are important in organic synthesis, providing a good way to form carbon-carbon bonds.
Cannizzaro reaction is given by only those aldehydes which do not contain alpha hydrogen, eg: benzaldehyde, formic acid, etc. Aldehydes which do not have an α-hydrogen atom, undergo self oxidation and reduction reaction on treatment with a concentrated alkali.
Compound A reacts with alcoholic KOH which gives compound B on further ozonolysis gives a compound C which does not contain an Alpha-H and compound D which contains an alpha-H. This sequence can be achieved by (B) and (C).
Since, compound A gives a white precipitate with silver nitrate, option C will be preferred, as tertiary alkyl reacts with silver nitrate more easily and the following reaction will take place;
Compound C gives only Cannizzaro reaction and compound D gives aldol condensation only.
The correct option is C.
Note: The possibility to make a mistake is that you may choose option B but the reaction of silver nitrate with the alkyl group more easily occurs in the tertiary group as compared to primary.