Question
Question: A compound A \(\left( {{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{\text{C}}{{\text{l}}_{\text...
A compound A (C5H10Cl2) on hydrolysis gives C5H10O which reacts with NH2OH, forms iodoform but does not give Fehling test A is
Solution
Fehling test is a characteristic test used to detect aldehydes. Thus, compounds other than aldehydes do not give Fehling test. The intermediate formed in the reaction does not give Fehling test and thus, it is not an aldehyde. Thus, the product formed in the reaction is a ketone. The intermediate reaction with NH2OH gives iodoform. Formation of iodoform is a characteristic test of methyl ketone (CH3−C=O) group. Thus, the intermediate form is a ketone with methyl (−CH3) group attached to it.
Complete step by step answer:
-A compound (A) has a molecular formula C5H10Cl2. This compound undergoes hydrolysis. Hydrolysis means that it reacts with H3O + and forms a compound with molecular formula C5H10O which reacts with NH2OH to give iodoform. The reaction is as follows:
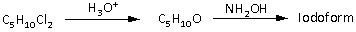
The compound with molecular formula C5H10O can be an aldehyde or a ketone. But it does not give the Fehling test. Fehling test is a characteristic test for aldehydes. Thus, the compound with molecular formula C5H10O is not an aldehyde. Thus, it is a ketone.
Also, C5H10O produces iodoform which is a characteristic of methyl ketone group. Thus, C5H10O is a ketone with methyl group attached to it. Thus, the structure is,
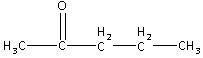
C5H10O is produced by the hydrolysis of C5H10Cl2. Thus, in C5H10O, the =O atom is replaced by two −Cl atoms. Thus, the structure of compound A (C5H10Cl2) is,
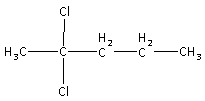
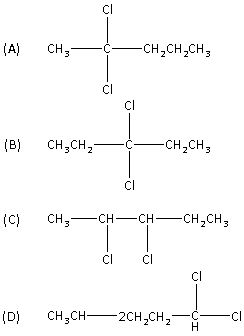
So, the correct answer is “Option A”.
Note: The compound gives Fehling test negative. Thus, it cannot be an aldehyde but can be a ketone. The compound on reaction with NH2OH gives iodoform. Iodoform is a characteristic test for methyl ketone groups. Thus, compound is a ketone containing methyl group.
