Question
Question: A compound 'A' formula of \(\text{ }{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\text{C}{{\text{l}...
A compound 'A' formula of C3H6Cl2 on reaction with alkali can give 'B' of formula C3H6O or 'C' of formula C3H4 . 'B' on oxidation gave a compound of the formula C3H6O2 . 'C' with dilute H2SO4 containing Hg2+ ion gave 'D’ of formula C3H6O, which with bromine and alkali gave the sodium salt of C2H4O2 . Then 'A' is:
A) CH3CH2CHCl2
B) CH3CCl2CH3
C) CH3ClCH2CH2Cl
D) CH3CH(Cl)CH2Cl
Solution
The haloalkanes when reacted with alkali can undergo the substitution reaction. The obtained product can be unsaturated hydrocarbon or the carbonyl compound (aldehyde or ketone).The aldehyde on further oxidation results in the carboxylic acid. The alkyne can be oxidised by the sulphuric acid HgSO4 as catalyst to form the carbonyl compound.
Complete step by step answer:
We will solve this question in reverse direction to get the reactant.
Let’s find out what is D). Aldehydes and ketones having at least one methyl group linked to the carbonyl carbon atom are easily oxidised by sodium hypochlorite solution Br2 in dil. Alkali to give haloform. Here we have got the CHOONa as the product of the haloform reaction.
-By reverse mechanism , the D) must be a simple ketone.
-Thus, D) is acetone. The haloform reaction of acetone is as shown below,
CH3-CO-CH3NaOBr CHBr3 + H-CO-ONa
Letts find out C) The alkynes can be converted into the aldehydes or ketone by the hydration of alkynes in the presence of dil.sulphuric acid and HgSO4 as a catalyst. Here, we know that the, alkyne have the general formula of C3H4 , thus it is three carbon containing alkyne. This alkyne on oxidation gives the acetone. Thus, compound C) is as shown below,
C3H4 Hg2+,H2SO4 CH3-CO-CH3
Therefore, C3H4 is a propyne. The structure of C) is,
CH≡C−CH3
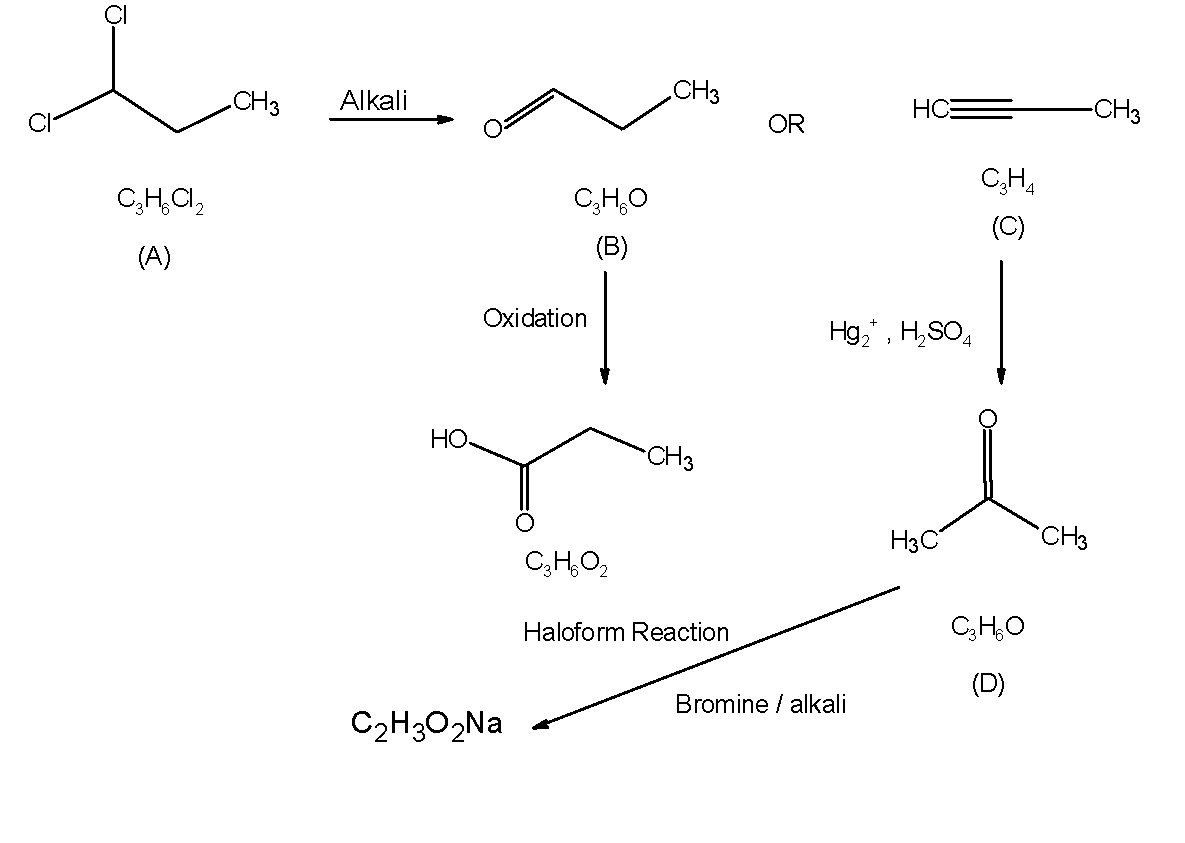
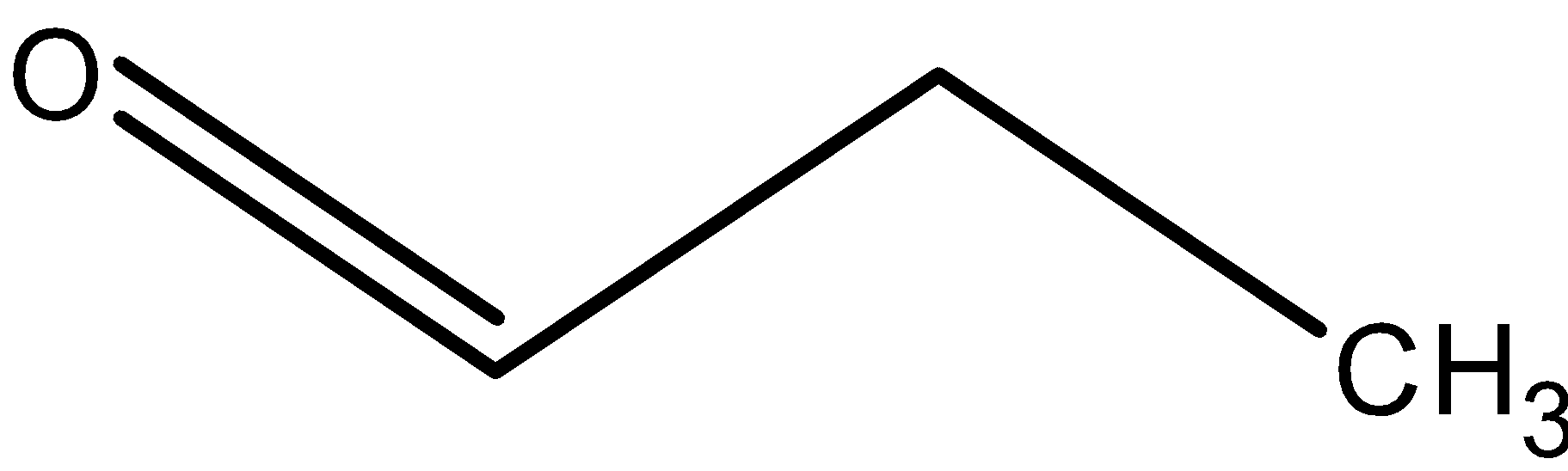
Now we will see what the compounds B) is It is found that the compound B) on further oxidation gives a compounds having formula C3H6O2 from the C3H6O. Thus the compound C3H6O can be alcohol, ketone or aldehyde and compound C3H6O2 is no doubt a carboxylic acid. We know that aldehydes are easily oxidised into the carboxylic acid in presence of oxidising agents. Thus compound B) is an aldehyde.
C3H6OOxidation C3H6O2
Thus, compound B) is as shown below,
Now, let’s find out compound A).
The compound have a general molecular formula as C3H6Cl2 . That is, two of the hydrogens are replaced by the chlorine atom. This can be on the same carbon atom or on the adjacent carbon atom. But, we are interested to obtain the aldehyde (propanaldehyde) from the dichloropropane, thus the compound should have the chlorine groups on the same carbon atom. Such that, the both chlorine are replaced by the hydroxyl groups from the alkali followed by the removal of water molecules. This results in the aldehyde. Thus, compound A) must have the chlorine on the same carbon atom. The structure is as follows,
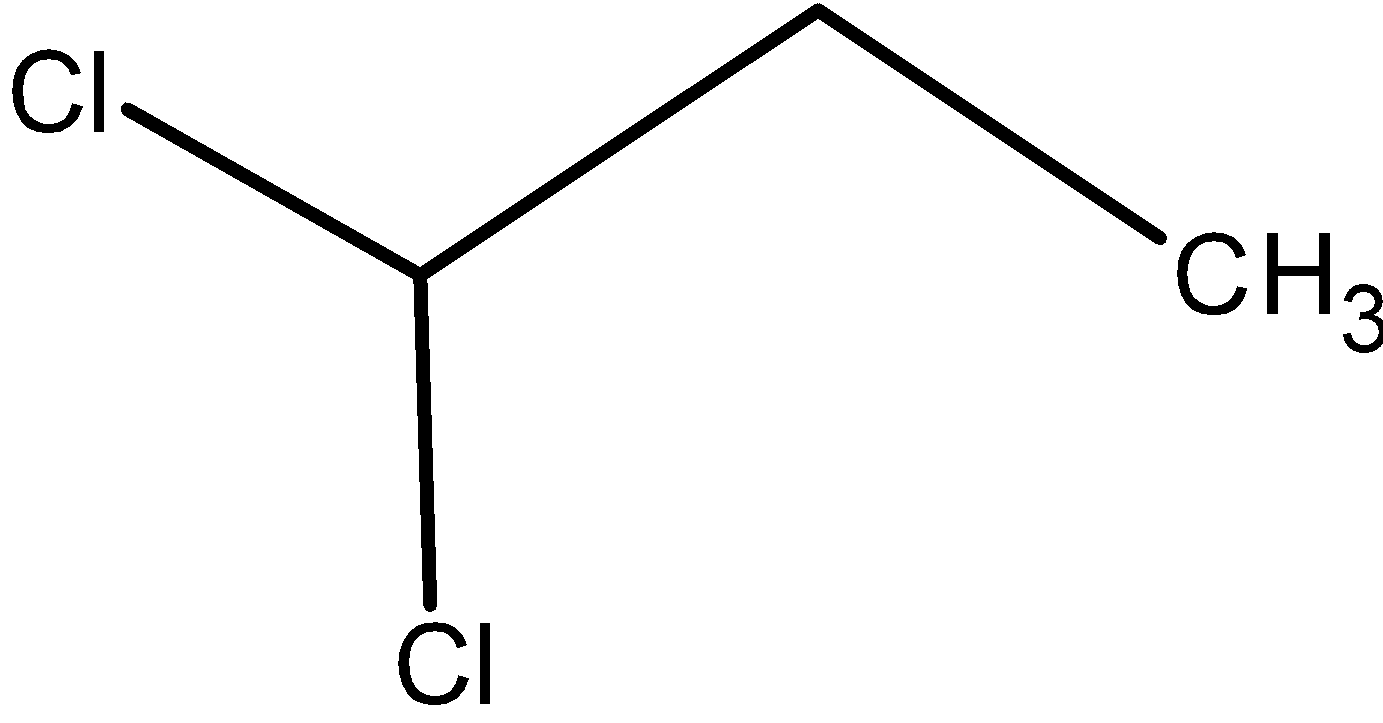
It is 1, 1 –dichloropropane.
Hence, (A) is the correct option.
Note: The diol form on the treatments of dichloropropane with the alkali are specifically geminal diol. These are the compounds in which the two hydroxyl groups are on the same carbon atoms. If they are considered to be on the adjacent carbon atoms (vicinal) then that would lead to the double bonded product which is unexpected. Thus, the reaction proceeds via gem diol synthesis.