Question
Question: A compound A \({C_2}{H_6}O\) on oxidation by PCC give B which on treatment with aqueous alkali and s...
A compound A C2H6O on oxidation by PCC give B which on treatment with aqueous alkali and subsequent heating gives C. B on oxidation by KMnO4 forms a monobasic carboxylic acid with molar mass 60g/mol . Identify A,B,C
Solution
PCC is pyridinium chlorochromate. It is a reagent that is used in organic synthesis for the oxidation of alcohol to Carbonyl groups. Carbonyl compounds are aldehydes and ketones. The reagent PCC mentioned in the question oxidizes primary alcohol to aldehydes. Potassium permanganate oxidizes carboxylic acid.
Complete step by step answer:
The given compound is C2H6O which is ethanol C2H5OH. Now pyridinium chlorochromate oxidizes it to aldehyde. As it is a primary alcohol, we get the product ethanal. So the product B is ethanal and A is ethanol.
The next step is the treatment of product B ( Ethanal) with Aqueous alkali. We know that an aldehyde on treatment with an aqueous alkali like NaOH undergoes aldol condensation that gives the product β−hydroxyaldehyde which on further heating gives α−β−unsaturatedaldehyde (unsaturated Is the presence of double bond).
The reaction is as follows
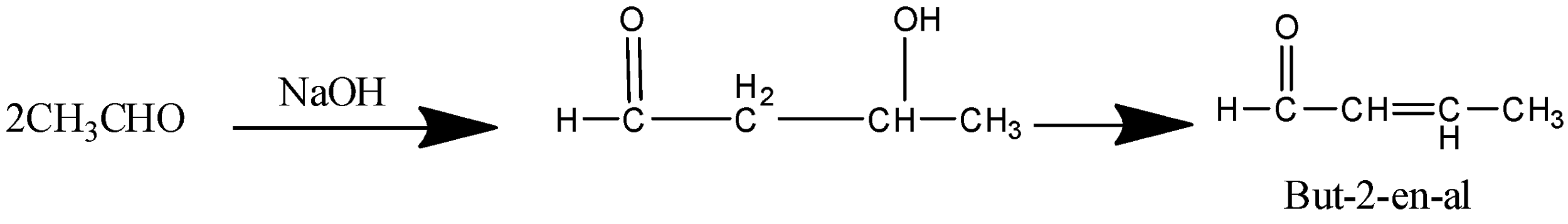
So the product C is But−2−en−al.
Now it is given that oxidation of B (acetaldehyde) with potassium permanganate gives the corresponding acid. We know that potassium permanganate is a strong oxidizing agent so it converts acetaldehyde into the corresponding acid that is ethanoic acid whose molar mass is 60g/mol .
Thus, compounds A, B, C are as follows.
A is Ethanol
B is Ethanal
C is But−2−en−al
The compound formed on subsequent heating of compound B with potassium permanganate is acetic acid (Ethanoic acid) which is having a molecular mass of sixty.
Note: PCC oxides primary alcohols to aldehydes and secondary alcohols to ketones. Potassium permanganate is a very strong oxidizing agent and it converts almost every compound with oxidizable hydrogen to an acid. The general formula for carboxylic acid is CnH2n+1COOH. Here n is the number of carbon atoms. Similarly, the general molecular formula of alcohol is CnH2n+1OH.
