Question
Question: A complex of the type \( {\left[ {M{{\left( {AA} \right)}_2}{X_2}} \right]^{n + }} \) is known to be...
A complex of the type [M(AA)2X2]n+ is known to be optically active. What does this indicate about the structure of the complex? Give one example of such a complex.
Solution
In order to answer the question let us first understand what optically active means. The capacity to rotate the plane of polarisation of a linearly polarised light is known as optical activity. This effect can only be seen in chiral materials, which lack mirror symmetry.
Complete answer:
When a molecule is chiral, it is the cause of its optical activity. The property demonstrated by substances in which the plane of polarisation is rotated for plane-polarized light is known as optical activity.
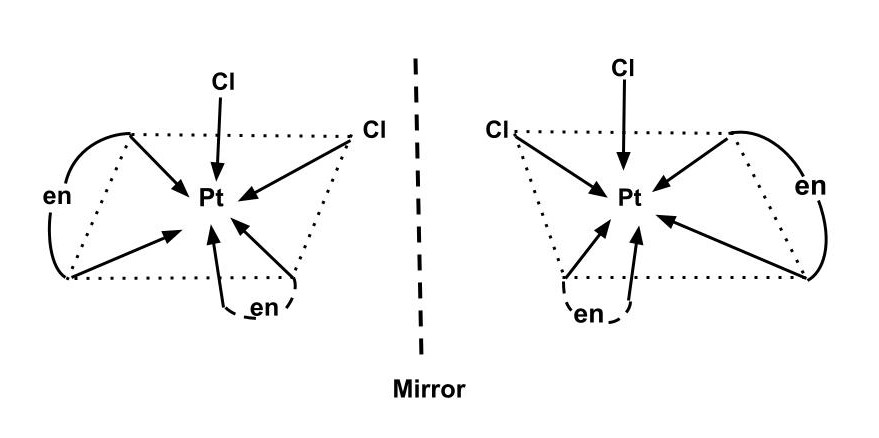
An optically active complex of the type [M(AA)2X2]n+ indicates cis-octahedral structure, e.g., cis−[Pt(en)2Cl2]2+ or cis−[Cr(en)2Cl2]+ because the mirror image isomers aren't superimposable.
Additional Information:
In chemistry, the words dextrorotation and laevorotation (sometimes spelled levorotation) are used to describe the optical rotation of plane-polarized light. Dextrorotation refers to clockwise or right-handed rotation from the observer's perspective, while laevorotation refers to counter clockwise or left-handed rotation.
Note:
It should be known that Dextrorotatory or dextrorotatory refers to a chemical compound that causes dextrorotation, whereas laevorotatory or laevorotatory refers to a chemical component that causes laevorotation. Compounds with these features are said to have optical activity since they are made up of chiral molecules.] A chiral molecule's enantiomer (geometric mirror image) will be laevorotatory if it is dextrorotatory, and vice versa. Enantiomers rotate plane-polarized light in opposite directions by the same amount of degrees.
