Question
Question: a. Complete the following chemical equations: i. \({\text{Xe}}{{\text{F}}_{\text{4}}} + {\text{Sb}...
a. Complete the following chemical equations:
i. XeF4+SbF6→
ii. Cl2+F2(excess)→
b. Explain each of the following:
i. Nitrogen is much less reactive than phosphorus.
ii. The stability of +5 oxidation state decreases down group 15.
ii. The bond angles (O−N−O) are not of the same value in NO2− and NO2+.
Solution
Hint: During the reaction of XeF4 with SbF5, migration of one fluorine ion takes place. The group 15 elements are nitrogen, phosphorus, arsenic, antimony and bismuth. The bond angle differs due to the presence of lone pairs of electrons.
**Complete step by step solution:
a. i. **When XeF4 reacts with SbF5 one fluorine atom from XeF4 migrates to SbF5. Thus, XeF4 is converted to XeF3 and SbF5 is converted to SbF6.
The reaction is as follows:
XeF4+SbF5→XeF3+SbF6
Thus, when XeF4 reacts with SbF5, XeF3 and SbF6 are formed.
ii. When Cl2 reacts with excess of F2, ClF3 is formed.
The reaction is as follows:
Cl2+F2(excess)→ClF3
Thus, when Cl2 reacts with F2, ClF3 is formed.
b. i. Nitrogen is a diatomic molecule. The two nitrogen atoms are bonded by a triple bond (N≡N).
Phosphorus is a tetratomic molecule. The four phosphorous atoms are bonded by single bonds.
It is difficult to cleave the nitrogen-nitrogen triple bond and more energy is required to break the bond.
Thus, nitrogen is much less reactive than phosphorus.
ii. The group 15 elements are nitrogen, phosphorus, arsenic, antimony and bismuth.
As we move down the group 15 of the periodic table, the tendency of the s-block electrons to participate in the chemical bonding decreases. This effect is known as inert pair effect.
The general electronic configuration for group 15 elements is ns2np3. The oxidation state exhibited by the group 15 elements is +3 and +5.
The stability of +5 oxidation state decreases down the group due to the inert pair effect and the stability of +3 oxidation state increases.
This is because the ns2 electrons are poorly shielded by the d- and f- electrons.
iii. The structures of NO2− and NO2+ are as follows:
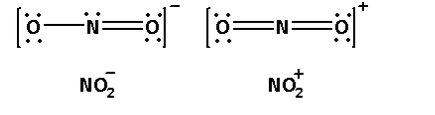
From the structures we can see that NO2− has one lone pair of electrons and NO2+ has no lone pair. Thus, the repulsion is higher in the case of NO2− and thus, its bond angle is lower than that of NO2+.
Thus, the bond angles (O−N−O) are not of the same value in NO2− and NO2+ due to the presence of lone pair of electron in NO2−.
Note:
XeF4 is known as xenon tetrafluoride. The geometry of xenon fluoride is octahedral because it has two lone pairs around the xenon atom. The general electronic configuration for group 15 elements is ns2np3. The stability of +5 oxidation state decreases due to the inert pair effect.
