Question
Question: A closed system can exchange: (A) matter with the surroundings (B) energy with the surroundings ...
A closed system can exchange:
(A) matter with the surroundings
(B) energy with the surroundings
(C) both A and B
(D) none of the above
Solution
The thermodynamics classify the system into three types as isolated system, closed system and the open system. All these systems have the contact with the surrounding for the exchange of the energy or the matter but I depend on the type of the system.
Complete step by step by step solution:
The energy is said to be either heat or work. The closed system is the system of the thermodynamics in which the energy of either heat or work can move between the system and the surrounding, but matter cannot be exchanged. Hence the closed system can 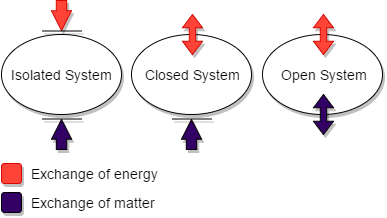
transmit only energy with the surrounding.
Thus the option (B) is correct.
Additional information:
The isolated system is the completely closed system that cannot exchange both the energy and the matter with the surrounding. The open system can transmit both of these with the surrounding. The matter implies the mass of the particles. The example of the open system is boiler, turbine, pump etc. The example of the isolated system is thermos flask, because it mains the mass inside it and also it does not get or release its heat.
Note: The example of the closed system is the earth. Because the earth gets the light energy from the sun but its mass remains constant without transmitting it to space. The other example of the closed system is the closed frying pan and the pressure cooker. This gets heat from the surrounding but the mass remains inside the frying pan itself.
