Question
Question: A closed container contains N molecules at P atm and T K. If the absolute temperature is doubled: ...
A closed container contains N molecules at P atm and T K. If the absolute temperature is doubled:
A.Number of molecules present in the container will become 2N.
B.Number of molecules present in the container will become N/2.
C.Gas pressure becomes 2P.
D.Gas pressure becomes P/2.
Solution
We have to know about Gay-Lussac’s law. We can state Gay-Lussac’s law as the pressure exerted by the gas is directly proportional to the absolute temperature at fixed mass and constant volume. The pressure of a gas at constant volume decreases constantly when it’s cooled until the gas undergoes the process of condensation and turns to a liquid. We can obtain a linear plot by graphing the relationship between pressure at constant volume and absolute temperature for a given mass of a gas.
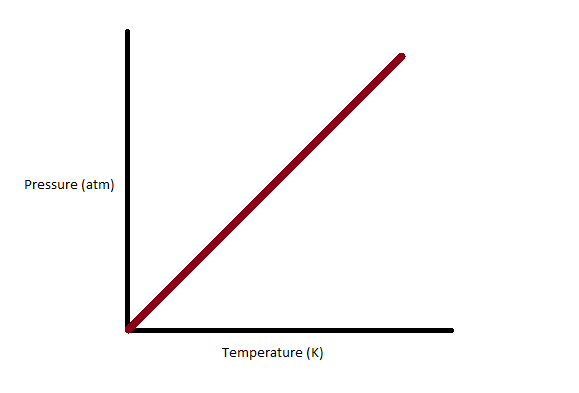
Complete step by step answer: At constant volume, for a fixed mass, the ratio of the initial temperature and pressure is equal to the ratio of final temperature and pressure is implied by Gay-Lussac’s law.
We can express the statement by using the formula,
(T1P1)=(T2P2)
Here, The initial pressure is expressed as P1.
The final pressure is expressed as P2.
The initial temperature is expressed as T1.
The final temperature is expressed as T2.
We know that for a given mass, at constant volume
PαT
We can write the proportionality constant expression for initial pressure and temperature as,
InitialtemperatureInitialpressure = constant T1P1 = k
We can write the proportionality constant expression for final pressure and temperature as,
FinaltemperatureFinalpressure = constant T2P2=k
Therefore, we can write the obtain expressions for initial pressure and temperature along with final pressure and temperature as,
T1P1=T1P2=k(or)P1T1=P2T2
A closed container contains the N number of molecules at Pressure P atm, temperature T K.
Since it is a closed container, the number of molecules would remain the same.
Let Pressure be P be the initial pressure.
T1P1=T2P2
The absolute temperature is doubled, then T2=2T1
The expression becomes,
T1P=2T1P2
Since, the temperature is doubled, P2=2P
The absolute temperature is doubled, the pressure of the gas will also be doubled.
∴Option C is correct.
Note:
Let us discuss an example of Gay-Lussac’s law
When we heat a pressurized aerosol such as deodorant can (or) can of spray-paint, there would be an increase in the pressure exerted by the gas on the container, this could lead into an explosion. This is the reason why several pressurized containers contain warning labels telling us why the container must be kept apart from fire and has to be stored in an environment that is cool.
