Question
Question: A chelating agent has two or more than two donor atoms to bind a single metal ion. Which of the foll...
A chelating agent has two or more than two donor atoms to bind a single metal ion. Which of the following is not a chelating agent?
A. Thiosulphato
B. Glycinato
C. Oxalato
D. Ethane −1,2− diamine
Solution
Chelating agent is defined as the chemical compound that basically reacts with metal ions so that it forms a stable water soluble complex. They are known by many names such as chelators and chelants. There are some chelating agents that bind iron and copper in blood and are used to treat high levels of these metals.
Complete step-by-step answer: Before talking about the answer, you should know the meaning of ligands. Ligands are defined as the ions or molecules that bind with central atom or ion and form a complex. In this complex, the ligand acts as a Lewis base and central atom acts as a Lewis acid.
Let us discuss the options one by one:
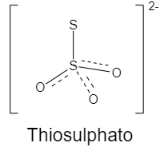
A. Thiosulphato is not a chelating agent as it does not have any coordination site. It is an ambidentate ligand.
Let us see the structure:
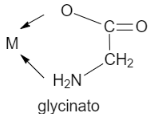
B. Glycinato is a chelating agent as it contains two coordinating site that are oxygen and nitrogen. It is a bidentate ligand.
Let us see the structure:
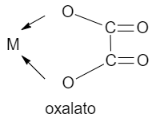
C. Oxalate is a chelating agent as it contains two coordinating sites that are two oxygen atoms. It is a bidentate ligand.
Let us see the structure:
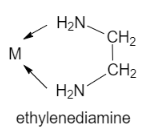
D. Ethane −1,2− diamine is a chelating agent as it contains two coordinating sites that are two nitrogen atoms donating their lone pair of electrons. It is a bidentate ligand.
Let us see the structure:
Note: It is to note that bidentate ligands are defined as the Lewis bases that donate two lone pairs of electrons to the central atom. They are referred to as chelating agents whereas ambidentate ligands are defined as the ligands that have the potential to donate electrons to more than one atom.
