Question
Question: A certain amount (say, $n$ moles) of a monatomic ideal gas ($C_v = 3/2R$) of a volume $V_1$ and pres...
A certain amount (say, n moles) of a monatomic ideal gas (Cv=3/2R) of a volume V1 and pressure P1 is expanded against a constant external pressure P2 until the pressure of the gas becomes P2. The correct statement is:
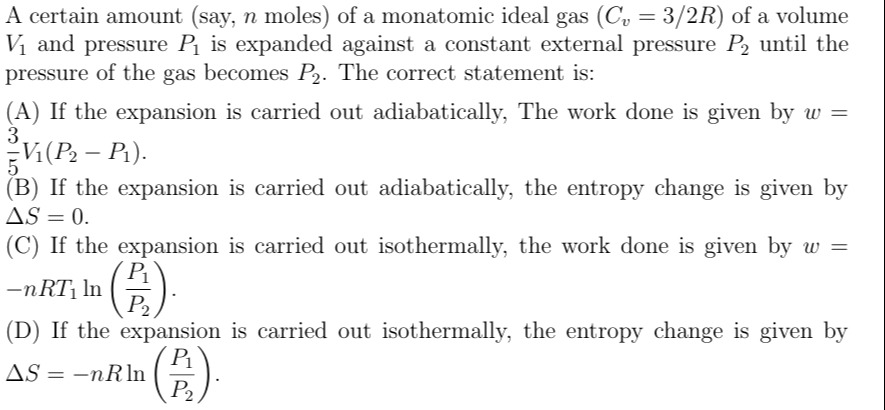
If the expansion is carried out adiabatically, The work done is given by w=53V1(P2−P1).
If the expansion is carried out adiabatically, the entropy change is given by ΔS=0.
If the expansion is carried out isothermally, the work done is given by w=−nRT1ln(P2P1).
If the expansion is carried out isothermally, the entropy change is given by ΔS=−nRln(P2P1).
If the expansion is carried out adiabatically, The work done is given by w=53V1(P2−P1).
Solution
The problem describes the expansion of a monatomic ideal gas against a constant external pressure P2 until the gas pressure also becomes P2. This is an irreversible process. We need to evaluate each statement.
Initial State: (P1,V1,T1) Final State: (P2,V2,T2) External Pressure: Pext=P2 (constant) Gas Type: Monatomic ideal gas, so Cv=23R.
Analysis of Option (A): If the expansion is carried out adiabatically, the work done is given by w=53V1(P2−P1).
- Process: Irreversible adiabatic expansion.
- First Law of Thermodynamics: ΔU=q+w.
- Adiabatic condition: q=0. So, ΔU=w.
- Change in Internal Energy for ideal gas: ΔU=nCvΔT=n(23R)(T2−T1).
- Work done against constant external pressure: w=−PextΔV=−P2(V2−V1).
- Equating ΔU and w: n(23R)(T2−T1)=−P2(V2−V1)
- Using Ideal Gas Law: P1V1=nRT1⟹T1=nRP1V1 and P2V2=nRT2⟹T2=nRP2V2.
- Substitute T1 and T2 into the equation from step 6: n(23R)(nRP2V2−nRP1V1)=−P2V2+P2V1 23(P2V2−P1V1)=−P2V2+P2V1 Multiply by 2: 3(P2V2−P1V1)=−2P2V2+2P2V1 3P2V2−3P1V1=−2P2V2+2P2V1 Rearrange to find V2: 3P2V2+2P2V2=2P2V1+3P1V1 5P2V2=(2P2+3P1)V1 V2=5P2(2P2+3P1)V1=52V1+53P2P1V1
- Substitute V2 back into the work equation: w=−P2(V2−V1)=−P2((52V1+53P2P1V1)−V1) w=−P2(52V1−V1+53P2P1V1) w=−P2(−53V1+53P2P1V1) w=53P2V1−53P1V1 w=53V1(P2−P1) This statement is correct. For an expansion, P1>P2, so (P2−P1) is negative, making w negative, consistent with work done by the system.
Analysis of Option (B): If the expansion is carried out adiabatically, the entropy change is given by ΔS=0.
- The process is an expansion against a constant external pressure, which is an irreversible process.
- For an irreversible adiabatic process, the entropy of the system increases, i.e., ΔSsys>0.
- ΔS=0 only for a reversible adiabatic process. This statement is incorrect.
Analysis of Option (C): If the expansion is carried out isothermally, the work done is given by w=−nRT1ln(P2P1).
- Process: Irreversible isothermal expansion.
- Work done against constant external pressure: w=−PextΔV=−P2(V2−V1).
- Isothermal condition: T1=T2=T.
- Using Ideal Gas Law: V1=P1nRT1 and V2=P2nRT1.
- Substitute V1 and V2 into the work equation: w=−P2(P2nRT1−P1nRT1) w=−nRT1(1−P1P2)=−nRT1(P1P1−P2) The expression given in the option, w=−nRT1ln(P2P1), is for a reversible isothermal expansion. This statement is incorrect.
Analysis of Option (D): If the expansion is carried out isothermally, the entropy change is given by ΔS=−nRln(P2P1).
- Process: Isothermal expansion. For an ideal gas, ΔSsys depends only on the initial and final states, regardless of the path (reversible or irreversible).
- Entropy change for an ideal gas: ΔS=nCvln(T1T2)+nRln(V1V2).
- Isothermal condition: T2=T1, so the first term is zero. ΔS=nRln(V1V2).
- Using Ideal Gas Law for isothermal process: P1V1=P2V2⟹V1V2=P2P1.
- Substitute into the entropy equation: ΔS=nRln(P2P1). The given expression is ΔS=−nRln(P2P1)=nRln(P1P2). Since it's an expansion, P1>P2, so P1/P2>1, and ln(P1/P2)>0. Thus, ΔS should be positive (entropy increases for expansion). The given expression with the negative sign would result in a negative ΔS, which is incorrect for an expansion. This statement is incorrect.
Final Conclusion: Only statement (A) is correct.
The final answer is A
Subject: Chemistry Chapter: Thermodynamics Topic: Work, Heat, Internal Energy, and Entropy Changes in Thermodynamic Processes (specifically Irreversible Processes for Ideal Gases)
