Question
Question: A carnot engine working between \(300K\) and \(600K\) has work output of \(800J\) per cycle. The amo...
A carnot engine working between 300K and 600K has work output of 800J per cycle. The amount of heat energy supplied to the engine from source per cycle will be:
A) 1000J
B) 1600J
C) 1200J
D) 900J
Solution
To solve this question we have use formula of efficiency of carnot engine which is given by η
η=Q1w=Q1Q1−Q2=T1T1−T2
Where w⇒ work done by substance
Q1⇒ Heat taken from source
Q2⇒ Remaining heat given to sink
T1⇒ Temperature of source
T2⇒ Temperature of sink
Step by step solution:
Step 1
In a carnot engine substance take heat or energy from a source which is at temperature T1 and change some amount of heat into work w and remaining heat transfer to sink at temperature T2 as shown in the diagram.
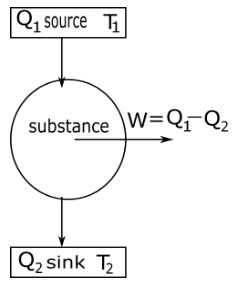
Efficiency of Carnot engine η defined as the ratio of net work done w by the engine during one cycle to the heat taken in from the source Q1 in one cycle thus.
⇒η=Q1w=Q1Q1−Q2
The efficiency of the Carnot engine depends on the absolute temperature of the sink and source. So
⇒η=Q1w=Q1Q1−Q2=T1T1−T2.......... (1)
From this equation
⇒Q1w=T1T1−T2
⇒Q1=w(T1−T2T1)
Step 2
Now we put all given value in this equation
⇒Q1=800(600−300600)
By solving this
⇒Q1=800(300600) ⇒Q1=800(2)
∴Q1=1600J
Hence we get the heat given by source is Q1=1600J
In this question the option B is correct.
Note:
The efficiency of a Carnot engine is given by ⇒η=Q1w=Q1Q1−Q2=T1T1−T2 we can find the efficiency of this engine
⇒η=Q1w=1600800=21
The percentage efficiency of the engine is ⇒η=21×100=50%
By this formula we can calculate all values associated with the Carnot engine.
