Question
Question: A carbonyl compound P, which gives positive iodoform test, undergoes reaction with \(C{{H}_{3}}MgBr\...
A carbonyl compound P, which gives positive iodoform test, undergoes reaction with CH3MgBr, followed by dehydration to give an olefin Q. The ozonolysis of Q gives rise to a dicarbonyl compound R, which undergoes intramolecular aldol condensation reaction to predominantly give S.
The structure of the carbonyl compound P is:
Solution
Methyl ketones respond to iodoform test and form respective carboxylic acid and iodoform as the products. It is a test used to find the presence of methyl ketones in the given compounds. Generally methyl ketones react with Grignard reagent and form respective alcohols as the products.
Complete answer:
- In the question it is mentioned that the compound-P gives Iodoform test, means compound-P must be a methyl ketone. Then from the given options P will be either A or B, because Option A and B only contains methyl ketones in their structure.
- Now by taking structures from option A and option B we will proceed and write the products formed as per the question. Later will decide which option will be correct.
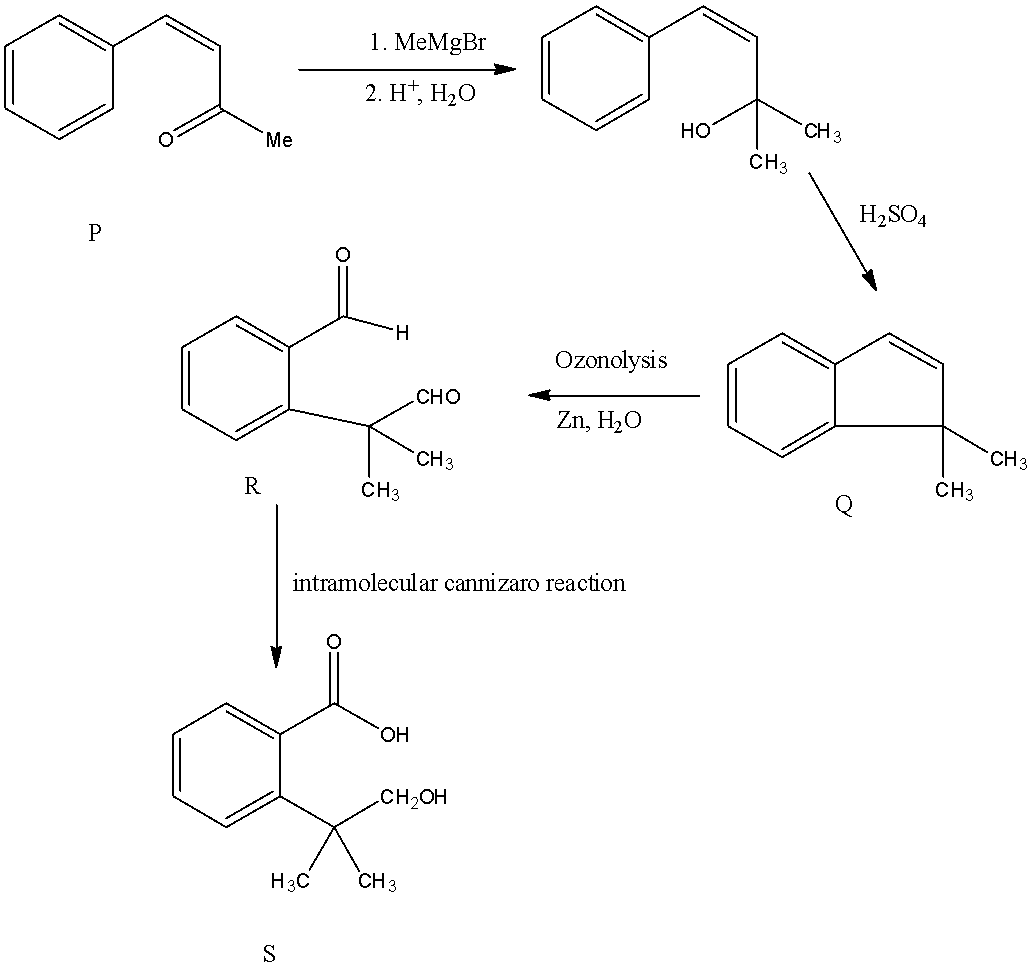
- If we are going to take Option A as P the following reaction takes place.
- If we are going to take Option B as P the following reaction takes place.
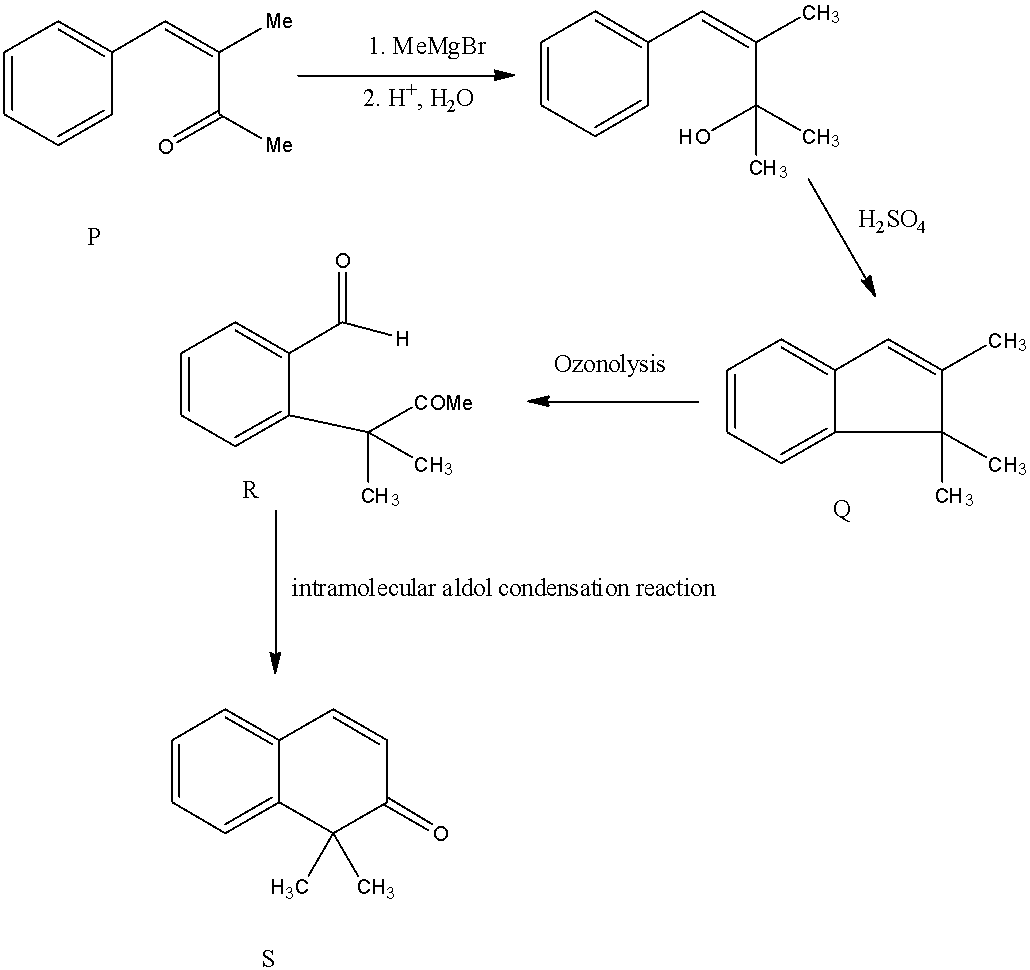
- But compound ‘R’ undergoes intramolecular aldol condensation and forms a cyclic compound (S), it only happens if we consider compound P is Option B.
- If we take compound P is option B, then in intermolecular aldol condensation it won’t form a cyclic compound (S)
Therefore the compound P is option B.
Note:
If we are going to take option A is compound P then the compound R formed as an intermediate product undergo intramolecular Cannizaro reaction and forms a carboxylic group and an alcohol in compound S. but in the question it is clearly mentioned the compound R undergoes intramolecular aldol condensation reaction means the Compound P is option B only.
