Question
Question: A buffer solution is prepared by mixing 'a' moles of CH3COONa and 'b' moles of CH3COOH such that (a ...
A buffer solution is prepared by mixing 'a' moles of CH3COONa and 'b' moles of CH3COOH such that (a + b) = 1, into water to make 1L buffer solution. If the instantaneous (differential) buffer capacity of this buffer solution is plotted against moles of salt CH3COONa(1) then the plot obtained will be (to the scale) approximately. (As shown in figure in options)
A

B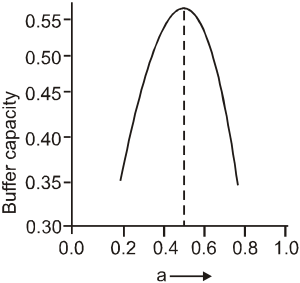

C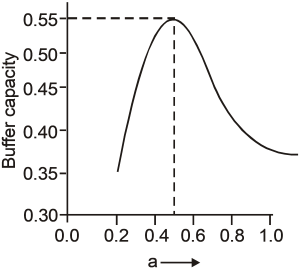

D

Answer

Explanation
Solution
Maximum buffer capacity of a solution is given by, buffer capacity = 2.303  . Hence the result.
. Hence the result.
and a = b = 0.5, BC = 2.303 × 1(0.5)2 = 0.57
