Question
Question: A big irregular shaped vessel contained water, conductivity of which was 2.56 × 10⁻³ S⁻¹m⁻¹. 585 g o...
A big irregular shaped vessel contained water, conductivity of which was 2.56 × 10⁻³ S⁻¹m⁻¹. 585 g of NaCl was then added to the water and conductivity after the addition of NaCl, was found to be 3.06 × 10⁻³ S⁻¹m⁻¹. The molar conductivity of NaCl at this concentration is 1.5 × 10⁻² S⁻¹m²mol⁻¹. The capacity of vessel if it is fulfilled with water, is
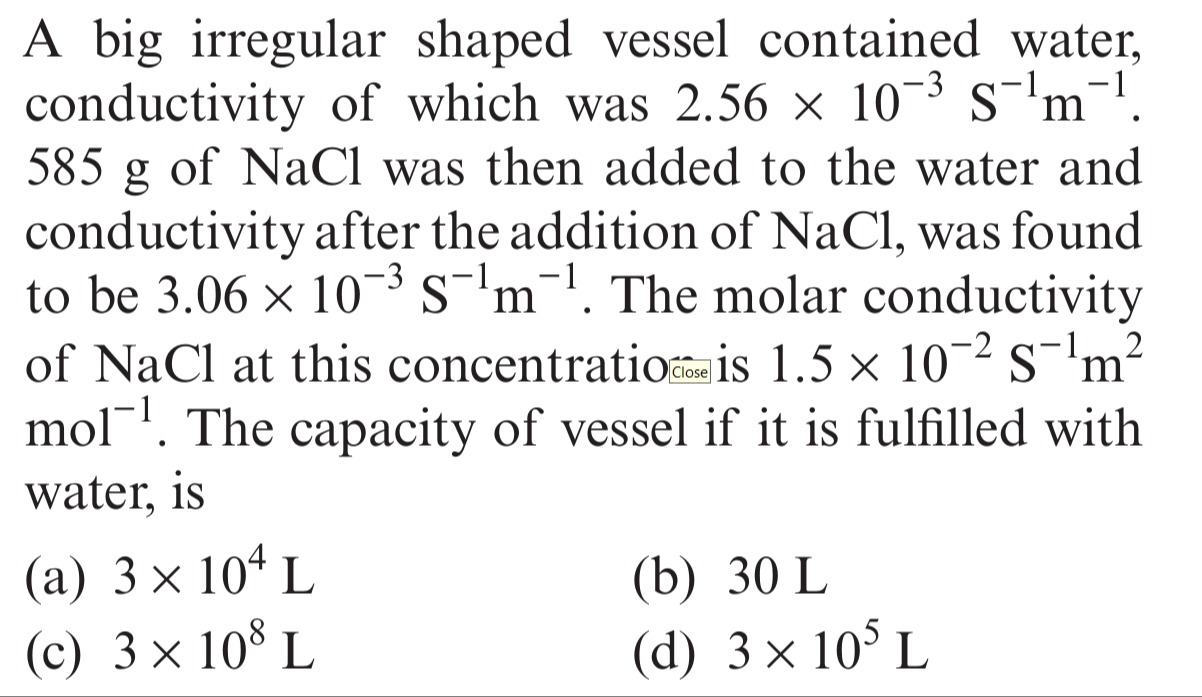
3 × 10⁴ L
3 × 10⁸ L
30 L
3 × 10⁵ L
3 × 10⁵ L
Solution
-
Calculate the conductivity due to NaCl: The conductivity of the solution is the sum of the conductivity of water and the conductivity contributed by NaCl. κNaCl=κsolution−κwater κNaCl=(3.06×10−3−2.56×10−3) S m−1=0.50×10−3 S m−1
-
Calculate the molar concentration of NaCl: The relationship between specific conductivity (κ), molar conductivity (Λm), and molar concentration (c) is κ=c×Λm. c=ΛmκNaCl c=1.5×10−2 S m2 mol−10.50×10−3 S m−1=1.50.50×10−1 mol m−3=31×10−1 mol m−3=301 mol m−3
-
Calculate the number of moles of NaCl: The molar mass of NaCl is 23.0 g/mol (Na)+35.5 g/mol (Cl)=58.5 g/mol. nNaCl=Molar mass of NaClMass of NaCl=58.5 g/mol585 g=10 mol
-
Calculate the volume of the solution: The molar concentration is moles of solute per unit volume of solution (c=n/V). Vsolution=cnNaCl Vsolution=301 mol m−310 mol=10×30 m3=300 m3
-
Convert the volume to Liters: 1 m³ = 1000 L. Vsolution=300 m3×1000 L/m3=300,000 L=3×105 L The capacity of the vessel is assumed to be equal to the volume of the solution formed.
