Question
Question: $\begin{array}{c} \mid \\ -C-O-C- \text{ bond angle in ethers is} \\ \mid \end{array}$ ...
∣−C−O−C− bond angle in ethers is∣
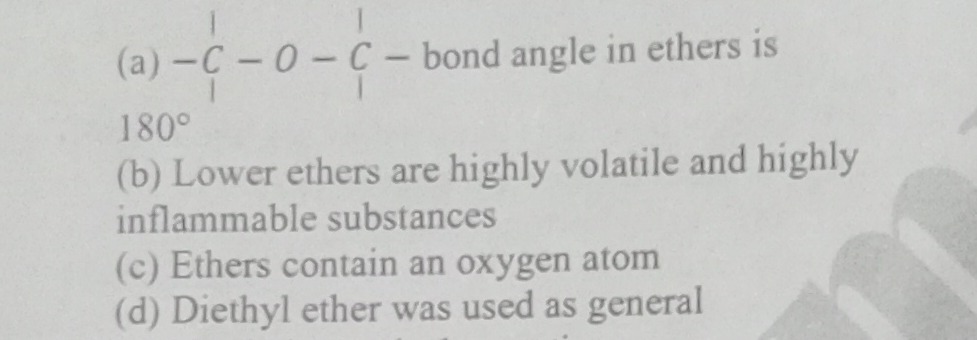
A
180°
B
Lower ethers are highly volatile and highly inflammable substances
C
Ethers contain an oxygen atom
D
Diethyl ether was used as general
Answer
The correct options are (b), (c), and (d).
Explanation
Solution
- (a) The –C–O–C– bond angle in ethers is around 110° due to the sp³ hybridization of oxygen with two lone pairs. Hence, 180° is incorrect.
- (b) Lower ethers are indeed volatile and highly inflammable.
- (c) By definition, ethers contain an oxygen atom bonded to two alkyl groups.
- (d) Diethyl ether was historically used as a general anesthetic.
(a) is false because the bond angle is approximately 110° (not 180°). (b), (c), and (d) are true based on the properties and historical use of ethers.
