Question
Question: (A), (B) and (C) are three non-cyclic functional isomers of a carbonyl compound with molecular formu...
(A), (B) and (C) are three non-cyclic functional isomers of a carbonyl compound with molecular formula C4H8O. Isomers (A) and (C) give positive Tollens’ test whereas isomer (B) does not give Tollens’ test but gives protection iodoform test. Isomers (A) and (B) on reduction with Zn(Hg)/HCl give the same product (D).
Out of (A), (B) and (C) isomers, which one is least reactive towards addition of HCN?
Solution
Tollen’s test is given only by aldehydes and not ketones. The iodoform test is given by compounds which have a methyl group attached to a carbonyl carbon in either an aldehyde or ketone. The reagent Zn(Hg)/HCl is Clemmensen’s reagent and reduces carbonyl carbon into methylene. The least reactive towards HCN would be a carbonyl compound with large groups as substituents.
Complete step by step answer:
The first clue given about the compounds (A), (B) and (C) is that they are aliphatic compounds, meaning they lack a ring structure. Their general formula is given as C4H8O. The three of them belong to the “but” family and are functional isomers of each other.
The second clue is about the Tollens’ test. This test is used to detect aldehyde in a mixture. A silver mirror is formed when ammoniacal silver nitrate ([Ag(NH3)2]+) solution (which is the chemical name of Tollens’ reagent) reacts with an aldehyde in an alkaline medium with exposure to heat. The chemical reaction is as below:
RCHO+2[Ag(NH3)2]++3OH−→RCOO−+2Ag+2H2O+4NH3
The aldehyde is converted into its corresponding carboxylate anion. Hence, this reaction can be characterized as an oxidation reaction. As only aldehydes are able to react, that proves the compounds (A) and (C) to be aldehydes.
The compound (B) does not give Tollens’ test but gives an iodoform test. The only compounds that react in this particular test have a methyl group attached to the carbonyl carbon. This makes compound (B) a methyl ketone.
The last clue tells us that reduction through Clemmensen’s process compounds (A) and (B) give the same compound. On treating an aldehyde or ketone with Zn(Hg)/HCl, the carbonyl oxygen atom is removed and two hydrogen atoms are added in its place.
Let’s start with compound (B). As it is already revealed it’s a methyl ketone which has a formula ofC4H8O. There is only one possible structure:
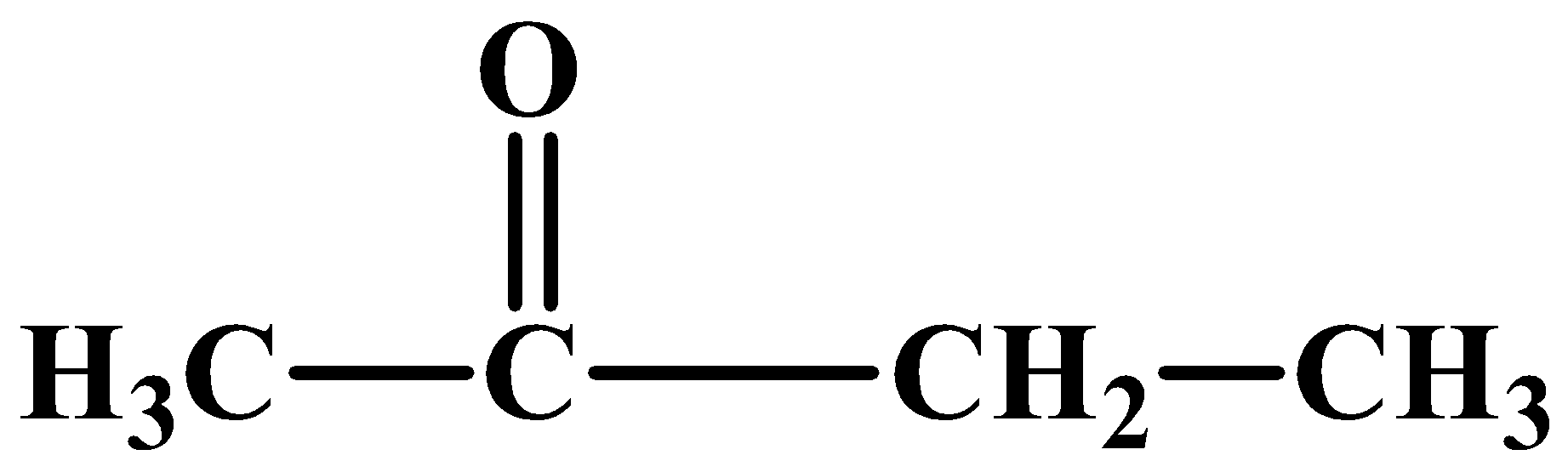
Compound (A) is an aldehyde and considering the other clues as discussed above, its structure can be:
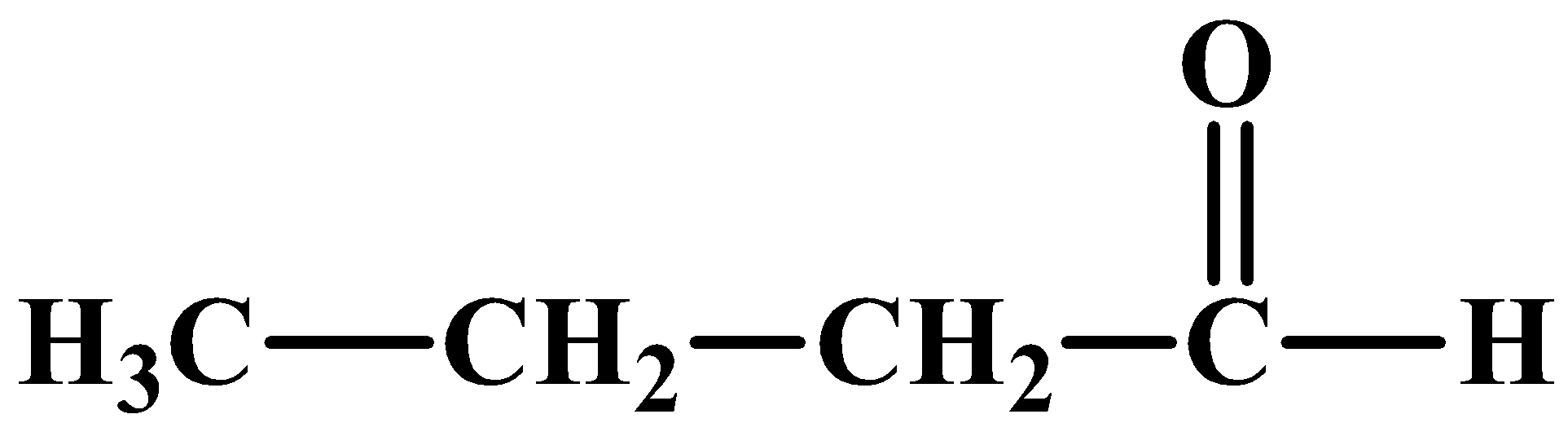
On reduction with Zn(Hg)/HCl, compound (A) and (B) give the same compound which is compound (D):
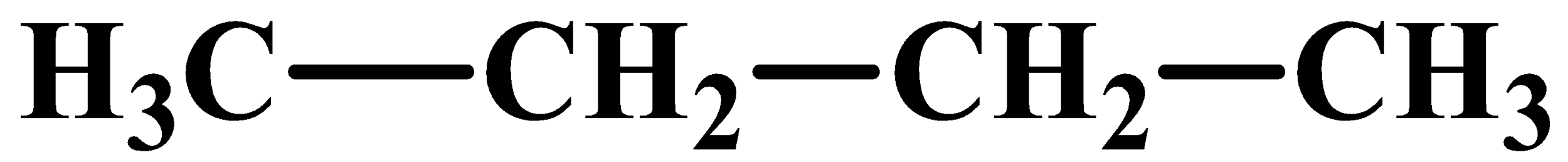 (Butane)
(Butane)
Now the last one, which is isomer (C) is also an aldehyde but different from that of (A), and so there is only one possible structure:
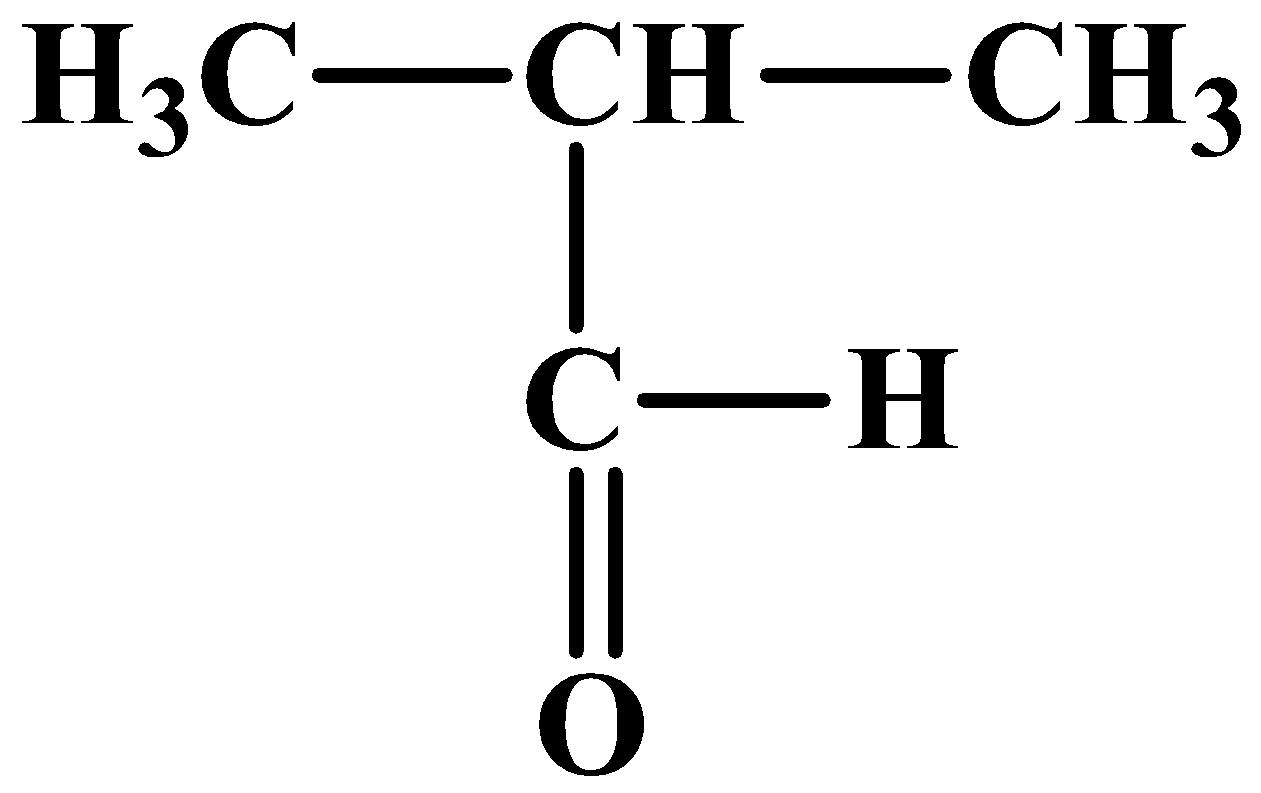
Aldehydes are the most reactive with hydrogen cyanide (HCN). So the least reactive among the compounds here is the ketone which is compound (B).
The reactions of the compounds that we have found out so far through our investigation is as below:
- Tollen’s test with compound (A)

- Tollen’s test with compound (C)
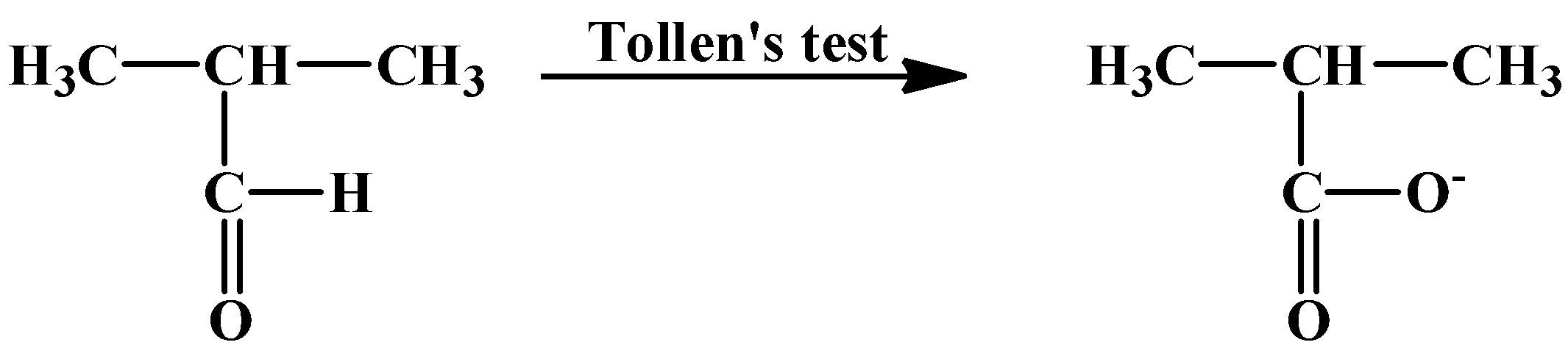
- Iodoform test with compound (B)
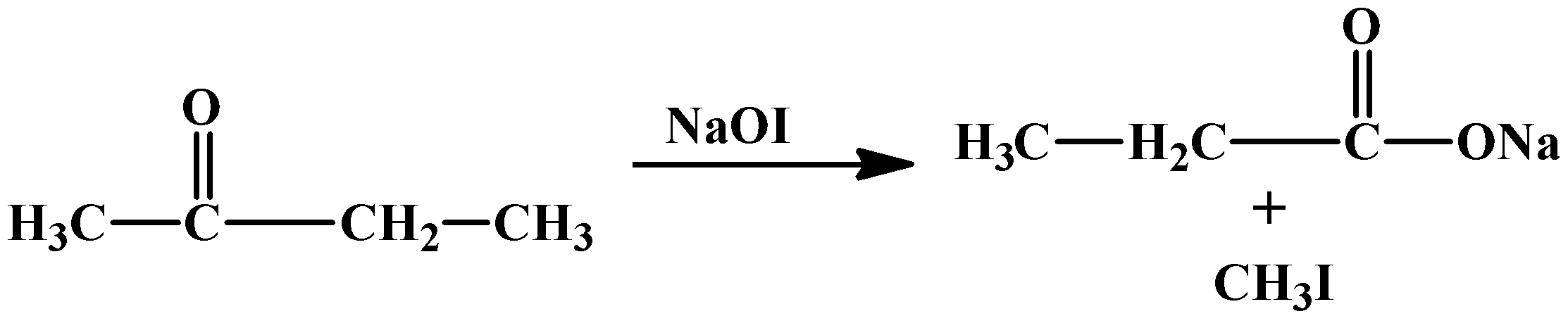
- Reduction of compound (A) and (B) with Clemmensen’s reagent
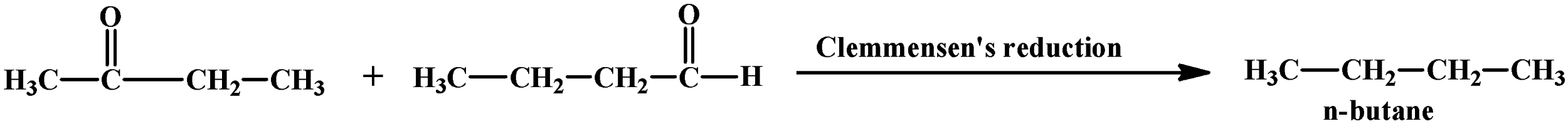
Note: In the above question, the reaction with HCN is actually a bimolecular nucleophilic addition reaction and therefore steric hindrance is a major factor. Compounds such as ketones where there are substituents on either side of the carbonyl carbon do not react readily with this reagent whereas aldehydes are relatively faster.
