Question
Question: \(A\) and \(B\) will be? 
Solution
This question gives us the knowledge about the cross cannizzaro reaction. Cross cannizzaro reaction is very useful in increasing the yield of the chemical compound. The compounds which contain α-hydrogen do not undergo a cannizzaro reaction.
Complete step-by-step answer:
Cross cannizzaro reaction is the reaction between two different aldehydes treated with a strong base, results in the formation of alcohol and a carboxylic acid. The compounds which contain α-hydrogen do not undergo a cannizzaro reaction.
Cannizarro reaction is a redox process because in this reaction both the oxidation reaction and the reduction reactions are taking place simultaneously. One aldehyde reduces itself to produce alcohol and the other aldehyde oxidizes itself to produce carboxylic acid. The main advantage of Cross cannizzaro reaction is that it helps in increasing the yield of the desired chemical compound.
The mechanism of cross cannizzaro reaction is as follows:
Firstly, the hydroxide ion HO−of the strong base acts as a nucleophile and attacks the carbonyl group of the benzaldehyde which leads to accumulation of negative charge on the oxygen atom. In the next step, hydride shift takes place which then attacks on the carbonyl group of formaldehyde HCHO. In the last step benzaldehyde is reduced to an alcohol and the formaldehyde is oxidized to the formic acid.
During this mechanism, the slowest step which is the rate determining step is the one in which hydride shift takes place.
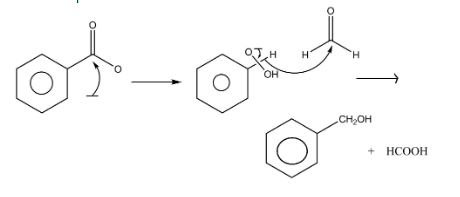
Therefore, the A is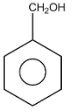 and B is HCOOH.
and B is HCOOH.
Note: You should always remember that cannizzaro reaction only takes place when there is no α- hydrogen is present in the chemical compound and is generally a redox reaction because oxidation reaction and reduction reactions are taking place simultaneously. Formaldehyde is the simplest aldehyde that undergoes cannizzaro reaction.
