Question
Question: (A) Account for the following: (i) Acidic character increases from \({\text{HF}}\) to \({\text{HI}...
(A) Account for the following:
(i) Acidic character increases from HF to HI.
(ii) There is a large difference between the melting and boiling points of oxygen and sulphur.
(iii) Nitrogen does not form pentahalide.
(B) Draw the structures of the following:
(i) ClF3
(ii) XeF4
Solution
Draw the structures of the molecules by counting the valence electrons for the molecule. The Lewis dot structures are also known as the electron dot structures. The electrons which do not participate in bonding are called lone pairs and the electrons which participate in bonding are called bond pairs.
Complete step by step answer:
(A)(i) In HI, the bond is formed between hydrogen and iodine and in HF bond is formed between hydrogen and fluorine.
The electronegativity of fluorine is more than that of iodine. Thus, the bond dissociation enthalpy of the bond between hydrogen and iodine is lower than that of the bond between hydrogen and fluorine.
Thus, the acidic character increases from HF to HI.
(ii) Oxygen is a diatomic molecule i.e. two atoms of oxygen are present in one molecule of oxygen (O2).
Sulphur is a polyatomic molecule i.e. eight atoms of sulphur are present in one molecule of sulphur (S8)
The melting and boiling points depend on the intermolecular forces of attraction.
Thus, there is a large difference between the melting and boiling points of oxygen and sulphur.
(iii) The atomic number of nitrogen is 7. Thus, the electronic configuration is 1s22s22p3.
The electronic configuration can be represented as follows:
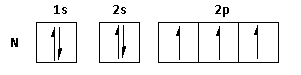
The valence electrons of nitrogen are 2sand 2p. Thus, nitrogen loses electrons from these valence orbitals and shows a +5 oxidation state.
But as there are no empty d-orbitals, nitrogen does not form pentahalide. Nitrogen can form trihalide.
(B)(i) Draw the structure of ClF3:
Calculate the valence electrons of ClF3 molecule as follows:
The valence electrons of chlorine are seven and fluorine are seven. Thus,
Valence electrons of ClF3 =(1×Valence electrons of Cl)+(3×Valence electrons of F)
=(1×7)+(3×7)
= 7 + 21
Valence electrons of ClF3 =28
Draw the Lewis structure of ClF3 as follows:
The structure of ClF3 is,
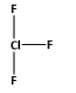
As three bonds are formed, six electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons =28−6=22
Place the remaining 22 electrons around the fluorine and chlorine atoms such that all the atoms complete their octets.
Thus, the structure of ClF3 is as follows:
(ii) Draw the structure of XeF4:
Calculate the valence electrons of XeF4 molecule as follows:
The valence electrons of xenon are eight and fluorine are seven. Thus,
Valence electrons of XeF4 =(1×Valence electrons of Xe)+(4×Valence electrons of F)
=(1×8)+(4×7)
= 8 + 28
= 36
Valence electrons of XeF4 = 36
Draw the Lewis structure of XeF4 as follows:
The structure of XeF4 is,
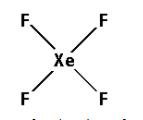
As four bonds are formed, eight electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons = 36 - 8 = 28
Place the remaining 28 electrons around the fluorine and xenon atoms such that all the atoms complete their octets.
Thus, the structure of XeF4 is as follows:
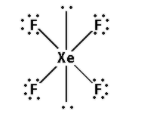
Note: An atom can form pentahalide when it has vacant d-orbital and it can accommodate five electrons of the halide. In case of nitrogen, d-orbitals are not present so that’s why nitrogen can’t form the pentahalides.
