Question
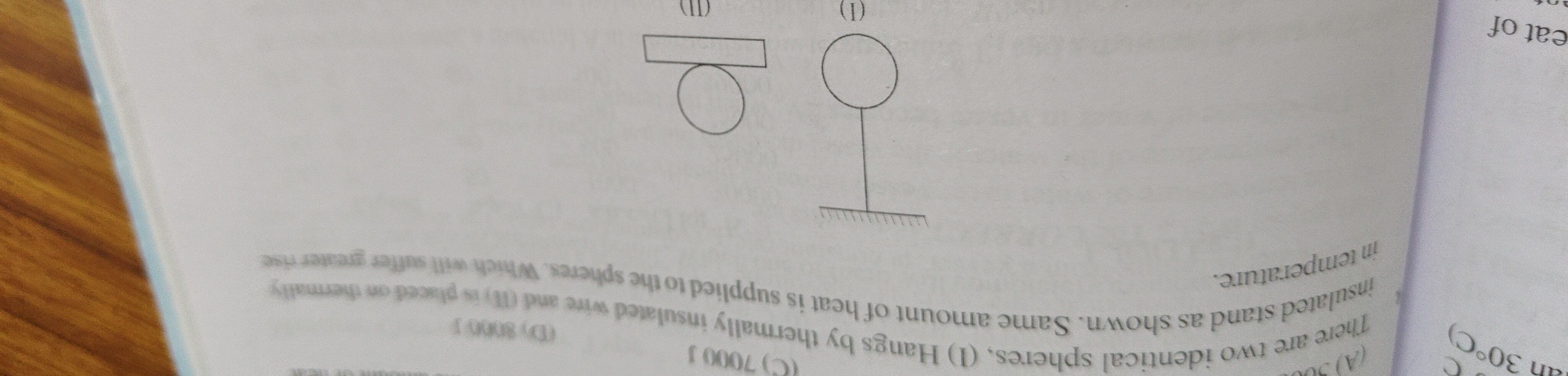
Question: There are two identical spheres, (I) Hangs by thermally insulated wire and (II) is placed on thermal...
There are two identical spheres, (I) Hangs by thermally insulated wire and (II) is placed on thermally insulated stand as shown. Same amount of heat is supplied to the spheres. Which will suffer greater rise in temperature.
500
7000 J
8000J
Sphere II
Sphere II
Solution
The rise in temperature (ΔT) of an object when heat (Q) is supplied is given by the formula Q=mcΔT, where m is the mass and c is the specific heat capacity. Thus, ΔT=mcQ.
We are given that the two spheres are identical, so they have the same mass (m) and the same specific heat capacity (c). The same amount of heat (Q) is supplied to both.
Sphere (I) hangs by a thermally insulated wire, so heat loss through the wire is minimized. Its entire surface area is exposed to the surroundings, leading to heat loss by convection and radiation. Sphere (II) is placed on a thermally insulated stand. The stand minimizes heat loss by conduction. However, the sphere is in contact with the stand, which reduces the surface area available for heat loss by convection and radiation compared to sphere (I).
Let A1 be the total surface area of sphere (I) and A2 be the exposed surface area of sphere (II). Due to the contact with the stand, A2<A1. The rate of heat loss is proportional to the exposed surface area. Therefore, the heat lost by sphere (II) (Qlost,II) is less than the heat lost by sphere (I) (Qlost,I).
The net heat absorbed by the sphere is Qnet=Qsupplied−Qlost. The temperature rise is ΔT=mcQnet. Since Qsupplied is the same for both spheres, and Qlost,II<Qlost,I, it follows that Qnet,II>Qnet,I. Consequently, ΔTII>ΔTI. Sphere (II) will suffer a greater rise in temperature.
