Question
Question: A \( {3^ \circ } \) alcohol as shown can be obtained by the reaction of ketone (diisopropyl ketone) ...
A 3∘ alcohol as shown can be obtained by the reaction of ketone (diisopropyl ketone) and _________.
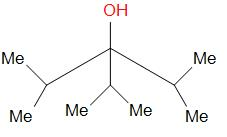
(A) isopropyl magnesium bromide
(B) isopropyl lithium
(C) diisopropyl cadmium
(D) diisopropyl zinc
Solution
Isopropyl magnesium Bromide, isopropyl lithium, diisopropyl cadmium, diisopropyl zinc; all give nucleophile. Nucleophile is a chemical species that donates an electron pair. They all replace the carbonyl bond with their alkyl group and OH functional group. Only the strongest nucleophile can add alkyl group to a sterically hindered compound.
Complete Step by step solution:
Isopropyl magnesium bromide is a Grignard reagent. The general formula for Grignard reagent of a Grignard compound is RMgX. Where R is an organic group, X is a halogen and Mg is magnesium. R can be an alkyl or aryl group. Grignard reagent helps in creating new carbon-carbon bonds.
Isopropyl lithium is an organolithium reagent as it contains carbon-lithium bonds. Organolithium reagents are important reagents in organic synthesis and they are frequently used to transfer organic group or lithium atoms to substrates through nucleophilic addition or simple deprotonation.
Diisopropyl cadmium is an organocadmium compound containing carbon to cadmium chemical bonds. Organocadmium compounds have the property of being sensitive to air, light and moisture. The first organocadmium compounds were dimethyl cadmium and diethyl cadmium prepared in 1917.
Diisopropyl zinc is an organozinc compound having two alkyl ligands. They are less reactive than Grignard reagent and organolithium reagent. They were among the first organometallic compounds made.
Among the following four compounds, organolithium compounds are more nucleophilic than Grignard, organocadmium compounds and organozinc compounds. The nucleophile released from organolithium compounds that is R− is sufficiently reactive to overcome steric hindrance.
The reaction that takes place is as follows,
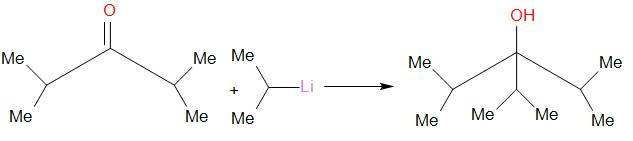
Hence, a 3∘ alcohol as shown can be obtained by the reaction of ketone (diisopropyl ketone) and isopropyl lithium.
Therefore, the correct answer is option B.
Note:
Organolithium compounds are not only good nucleophiles but also strong bases like Bu-Li. Grignard reagents are used for the conversion of carbonyls to alcohols.
