Question
Question: A \({{2}^{0}}\) alcohol as shown can be obtained by the reaction of di-t-butyl ketone and: a.) Is...
A 20 alcohol as shown can be obtained by the reaction of di-t-butyl ketone and:
a.) Isopropyl magnesium bromide
b.) t-butyl magnesium bromide
c.) EtMgBr
d.) MeMgBr
Solution
Secondary alcohol is a compound in which the hydroxyl group that is a −OH group is attached to a saturated carbon which also has two other carbons attached to it. A saturated carbon is a carbon which is bonded to other atoms by a single bond.
Complete step by step answer:
The di-t-butyl group is a bulky group and due to the steric hindrance of the di-t-butyl group in ketone the group containing bulky group or the group having beta hydrogen does not react with it which means that (30) tertiary alcohol cannot be obtained.
But if there is transfer of hydride ion i.e. H− from the beta position of the RMgX group to the C=Ogroup takes place then there is formation of cyclic transition state which produce secondary alcohol and alkene.
Hence MeMgBr does not react with di-t-butyl ketone which means that it will not produce secondary alcohol and alkene.
Reactions involves is shown below:
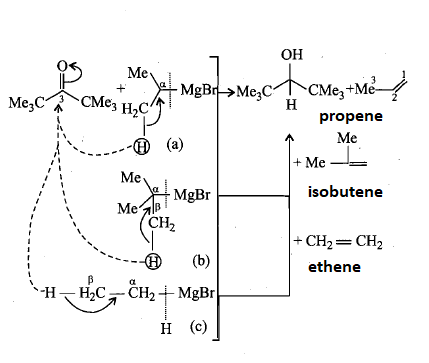
Hence the correct answer is option (A), option (B) and option (C) i.e. A 20 alcohol can be obtained by the reaction of di-t-butyl ketone and, Isopropyl magnesium bromide, t-butyl magnesium bromide, EtMgBr.
So, the correct answer is “Option A,B and C”.
Note: Alcohol is a homologous series having the functional group −OH also known as hydroxyl group. The general molecular formula for alcohols is CnH2n+1OH. The secondary alcohol isopropanol is a very important chemical which is used for the sustainable production of fuels and some other substances. Secondary alcohol can be oxidized to aldehydes and ketones using jones reagent.
