Question
Question: A 0.1 M solution of a metal salt (50 mL) reacts with 25 mL of 0.1 M sodium sulphite. Given the half-...
A 0.1 M solution of a metal salt (50 mL) reacts with 25 mL of 0.1 M sodium sulphite. Given the half-reaction
SO32− + H2O → SO42− + 2H+ + 2e−
If the metal starts with oxidation number +2, what is its oxidation number after reaction.
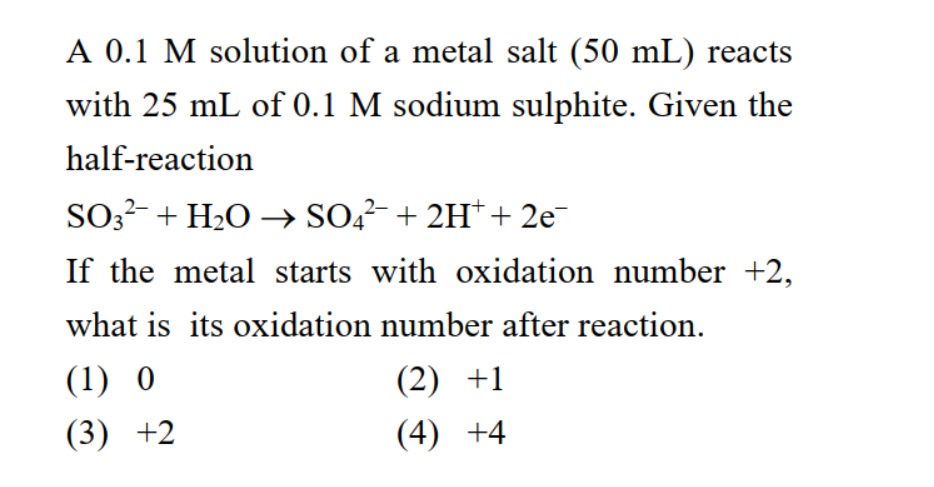
A
0
B
+1
C
+2
D
+4
Answer
+1
Explanation
Solution
-
Calculate moles of reactants:
- Moles of metal ions (M2+) = Molarity × Volume = 0.1M×50mL=0.1mol/L×0.050L=0.005mol.
- Moles of sulfite ions (SO32−) = Molarity × Volume = 0.1M×25mL=0.1mol/L×0.025L=0.0025mol.
-
Calculate moles of electrons transferred:
- The given half-reaction is SO32− + H2O → SO42− + 2H+ + 2e−.
- This shows that 1 mole of SO32− produces 2 moles of electrons.
- Therefore, 0.0025 mol of SO32− produces 0.0025mol×2=0.005mol of electrons.
-
Determine electron transfer per metal ion:
- The metal starts with an oxidation number of +2 (M2+).
- The electrons produced by the oxidation of sulfite are accepted by the metal ions, causing them to be reduced.
- We have 0.005 mol of M2+ ions and 0.005 mol of electrons available for reduction.
- Number of electrons accepted per M2+ ion = Moles of M2+Moles of e−=0.005mol0.005mol=1e−.
-
Calculate the final oxidation state of the metal:
- The metal ion starts with an oxidation state of +2.
- It accepts 1 electron during the reaction.
- The reduction half-reaction for the metal is M2++1e−→M+.
- The final oxidation state of the metal is +2 - 1 = +1.
