Question
Question: The major reason that phenol is a stronger Bronsted acid than cyclohexanol is...
The major reason that phenol is a stronger Bronsted acid than cyclohexanol is
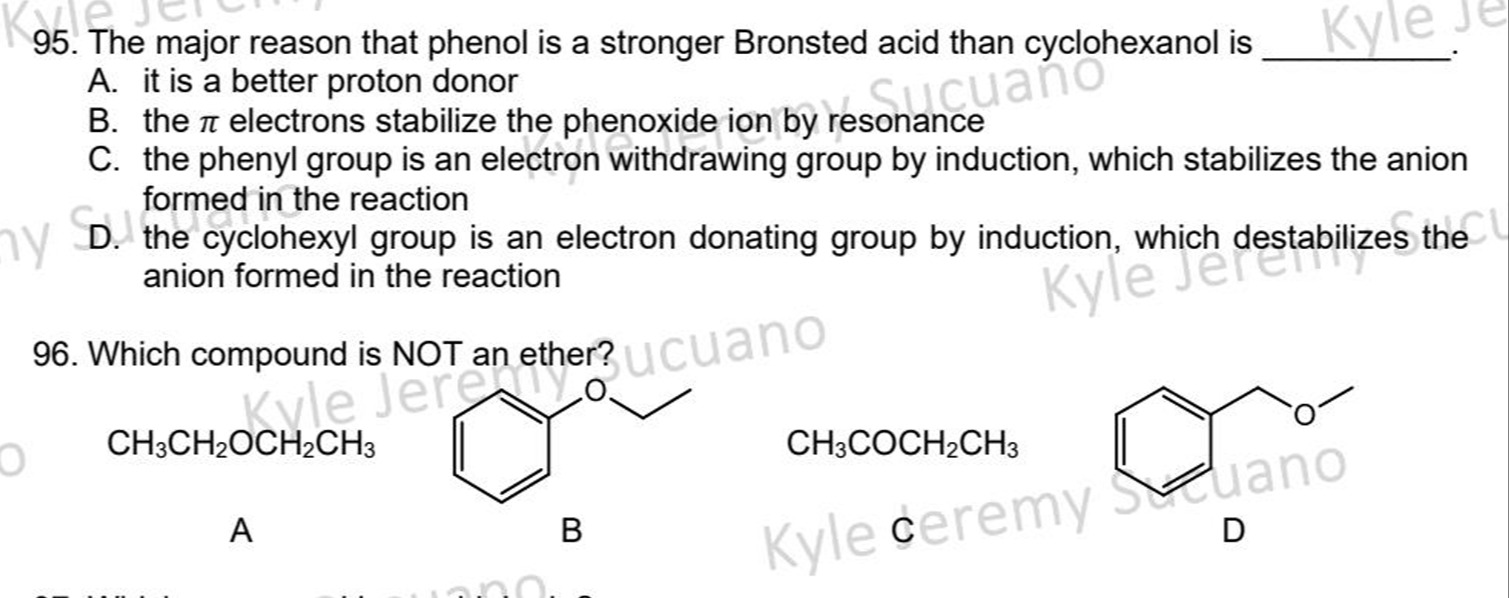
it is a better proton donor
the π electrons stabilize the phenoxide ion by resonance
the phenyl group is an electron withdrawing group by induction, which stabilizes the anion formed in the reaction
the cyclohexyl group is an electron donating group by induction, which destabilizes the anion formed in the reaction
the π electrons stabilize the phenoxide ion by resonance
Solution
The acidity of a Bronsted acid is determined by the stability of its conjugate base. When phenol loses a proton, it forms the phenoxide ion, where the negative charge on oxygen is delocalized into the benzene ring through resonance. This resonance stabilization significantly increases the stability of the phenoxide ion. In contrast, when cyclohexanol loses a proton, it forms the cyclohexoxide ion. The cyclohexyl group is electron-donating by induction, which pushes electron density towards the negatively charged oxygen, destabilizing the ion. The resonance stabilization of the phenoxide ion is the major factor making phenol a stronger acid than cyclohexanol.
