Question
Question: Which of the following compounds exhibits zero oxidation state of iron?...
Which of the following compounds exhibits zero oxidation state of iron?
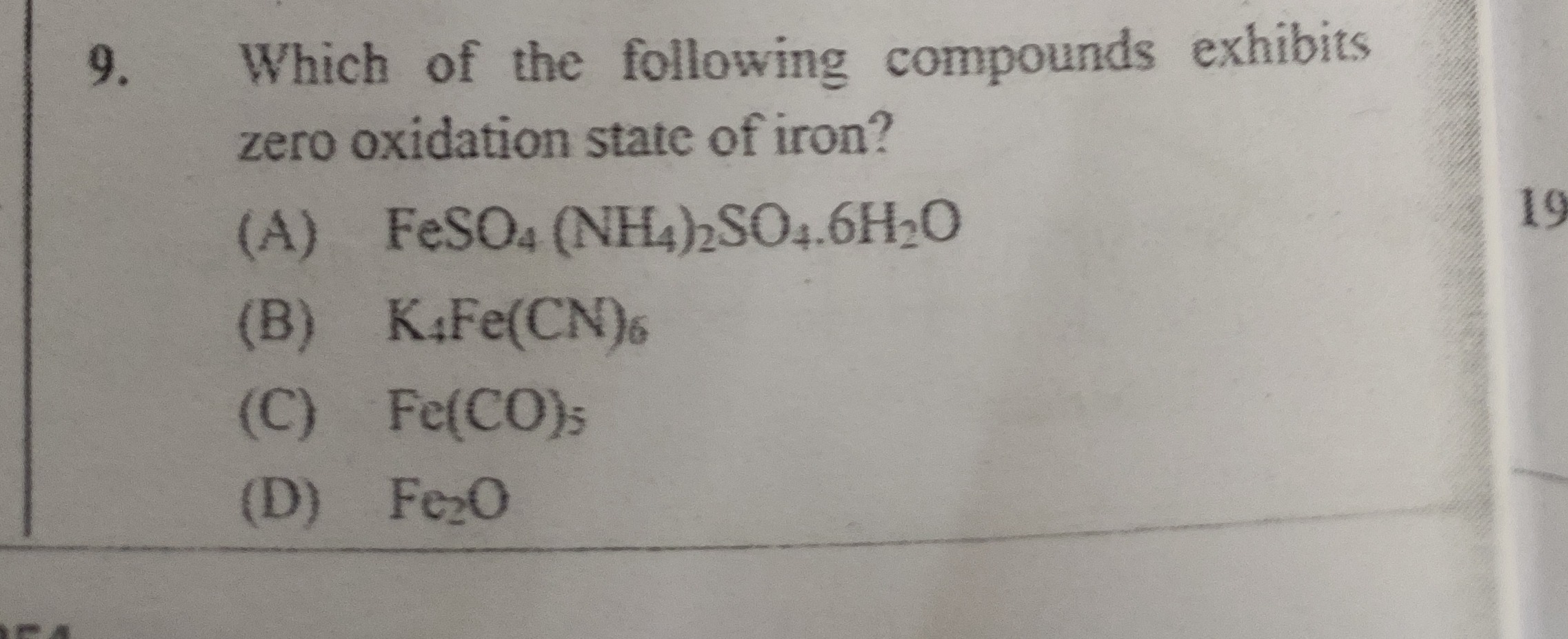
A
FeSO₄(NH₄)₂SO₄.6H₂O
B
K₄Fe(CN)₆
C
Fe(CO)₅
D
Fe₂O
Answer
Fe(CO)₅
Explanation
Solution
In Fe(CO)5, each CO is a neutral ligand. Hence, the oxidation state of iron is 0.
