Question
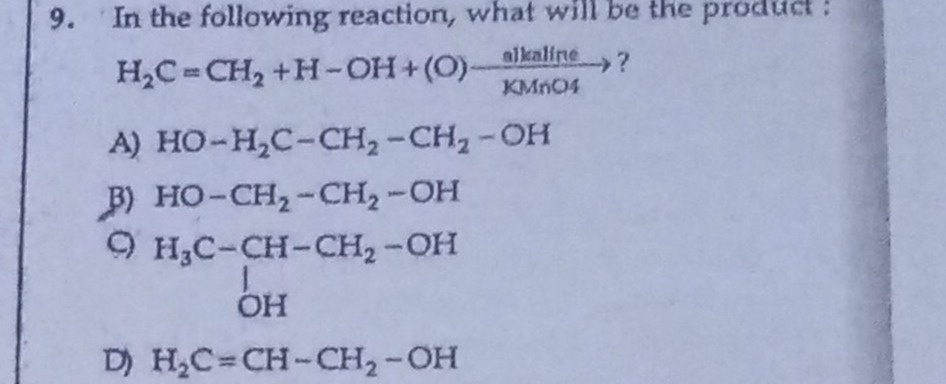
Question: In the following reaction, what will be the product: $H_2C=CH_2 + H-OH + (O) \xrightarrow[KMnO4]{al...
In the following reaction, what will be the product:
H2C=CH2+H−OH+(O)alkalineKMnO4?
A
HO-H2C−CH2−CH2−OH
B
HO-CH2−CH2−OH
C
H3C−∣CH−CH2−OH OH
D
H2C=CH−CH2−OH
Answer
HO‑CH₂‑CH₂‑OH
Explanation
Solution
Under cold, dilute alkaline KMnO₄ conditions, ethene undergoes syn-dihydroxylation, adding two –OH groups across the double bond to form ethylene glycol.
