Question
Question: Find the total number of 5-membered rings present in [Co(EDTA)]-....
Find the total number of 5-membered rings present in [Co(EDTA)]-.
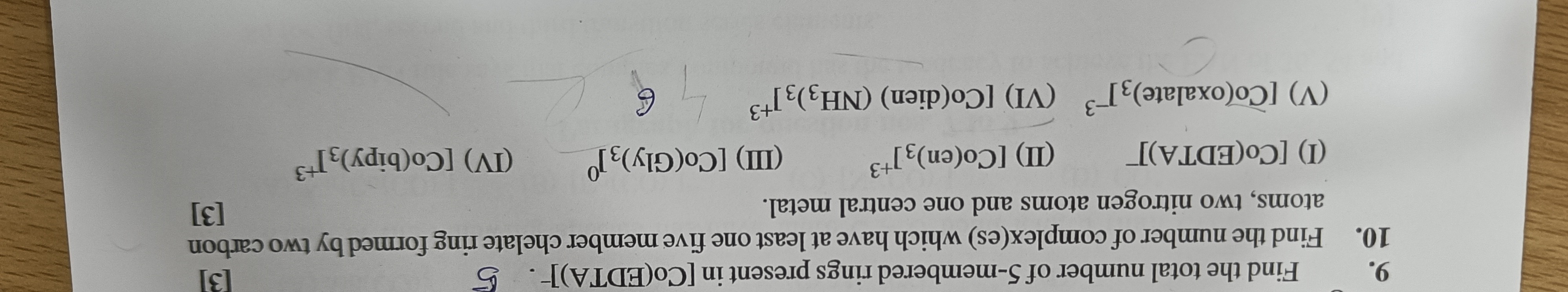
Answer
5
Explanation
Solution
The complex [Co(EDTA)]- contains one hexadentate EDTA ligand. EDTA coordinates through two nitrogen atoms and four carboxylate oxygen atoms. The structure of EDTA allows for the formation of 5 chelate rings with the central metal ion. One ring is formed by the ethylenediamine backbone (M-N-C-C-N) and is 5-membered. The other four rings are formed by the four acetate arms (M-N-C-C-O) and are also 5-membered. The total number of 5-membered rings is the sum of rings from the backbone and the arms.
Total 5-membered rings = 1 (from ethylenediamine backbone) + 4 (from acetate arms) = 5.
