Question
Question: A lump of ice containing a zinc pellet of mass m = 35 g is floating in a cylindrical vessel of botto...
A lump of ice containing a zinc pellet of mass m = 35 g is floating in a cylindrical vessel of bottom area S = 100 cm2. If all the ice melts, by what amount the water level in the vessel change? Density of zinc is ρz = 7000 kg/m3 and that of water is ρ = 1000 kg/m3.
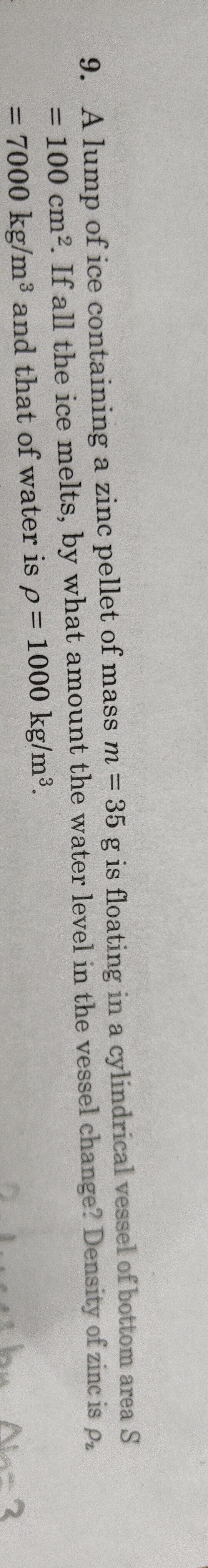
The water level will decrease by 0.3 cm.
Solution
When the ice melts, the total volume of water increases by the mass of the ice divided by the density of water. The zinc pellet, initially supported by the ice, will sink to the bottom, displacing a volume of water equal to its own volume. The change in water level is due to the difference in the volume displaced by the zinc pellet when it was floating (as part of the ice lump) and the volume it occupies when it sinks.
Let mz be the mass of the zinc pellet, ρz be its density, and ρw be the density of water. The volume of the zinc pellet is Vz=ρzmz. When the ice-zinc system floats, the weight of the zinc pellet is supported by a buoyant force. The volume of water displaced by the zinc pellet alone (if it were submerged to the same extent it is in the floating ice) would be Vdisp_z=ρwmz. When the ice melts, the zinc pellet sinks and displaces its own volume Vz. The change in the volume of water displaced is ΔV=Vz−Vdisp_z=ρzmz−ρwmz. The change in water level is Δh=SΔV.
Given: mz=35 g =0.035 kg S=100 cm2=0.01 m2 ρz=7000 kg/m3 ρw=1000 kg/m3
ΔV=7000 kg/m30.035 kg−1000 kg/m30.035 kg ΔV=5×10−6 m3−35×10−6 m3 ΔV=−30×10−6 m3
Δh=0.01 m2−30×10−6 m3=−3000×10−6 m=−0.003 m Δh=−0.3 cm.
The water level decreases by 0.3 cm.
