Question
Question: Identify most acidic hydrogen in given compound....
Identify most acidic hydrogen in given compound.
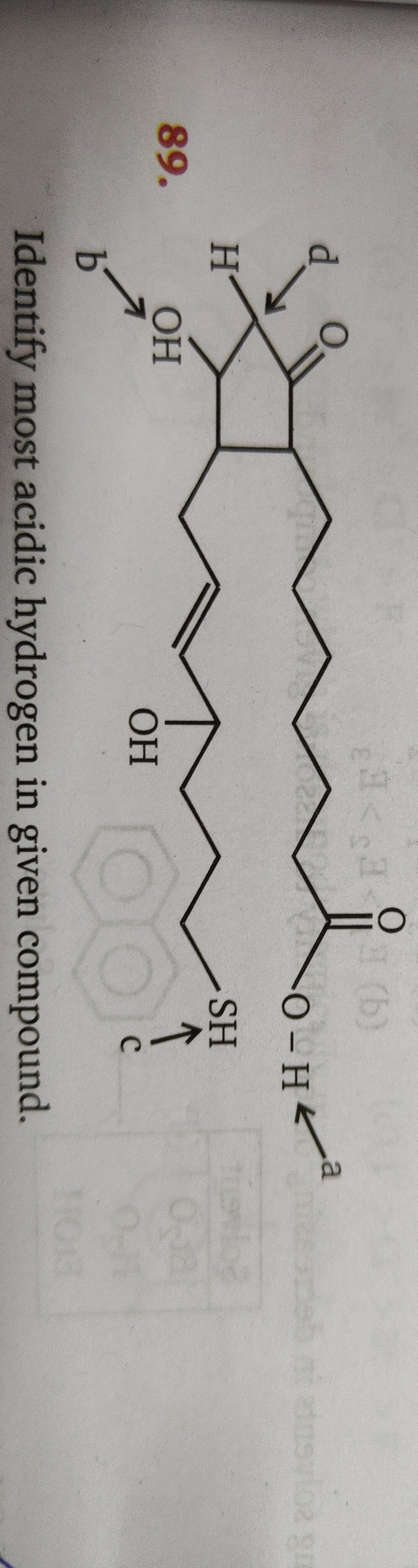
A
a
B
b
C
c
D
d
Answer
a
Explanation
Solution
The acidity of a hydrogen atom is determined by the stability of the conjugate base formed upon its removal. Hydrogen 'a' is part of a carboxylic acid group (-COOH), forming a resonance-stabilized carboxylate anion. Hydrogen 'c' is part of a thiol group (-SH), forming a thiolate anion, stabilized by sulfur's polarizability. Hydrogen 'b' is part of an alcohol group (-OH), forming an alkoxide anion, which is less stable. Hydrogen 'd' is an alpha-hydrogen to a ketone, forming an enolate anion, stabilized by resonance. Comparing stabilities: carboxylate > thiolate > enolate > alkoxide. Thus, hydrogen 'a' is the most acidic.
