Question
Question: Identify each species in the following equilibrium according to the code: SA = stronger acid; SB = s...
Identify each species in the following equilibrium according to the code: SA = stronger acid; SB = stronger base; WA = weaker acid; WB = weaker base. The pKₐ of (CH₃)₂NH is 36; the pKₐ of CH₃OH is 15.2.
CH₃OH + (CH₃)₂NH <=> + CH₃-O⁺-CH₃-NH-CH₃ 1 2 1 2
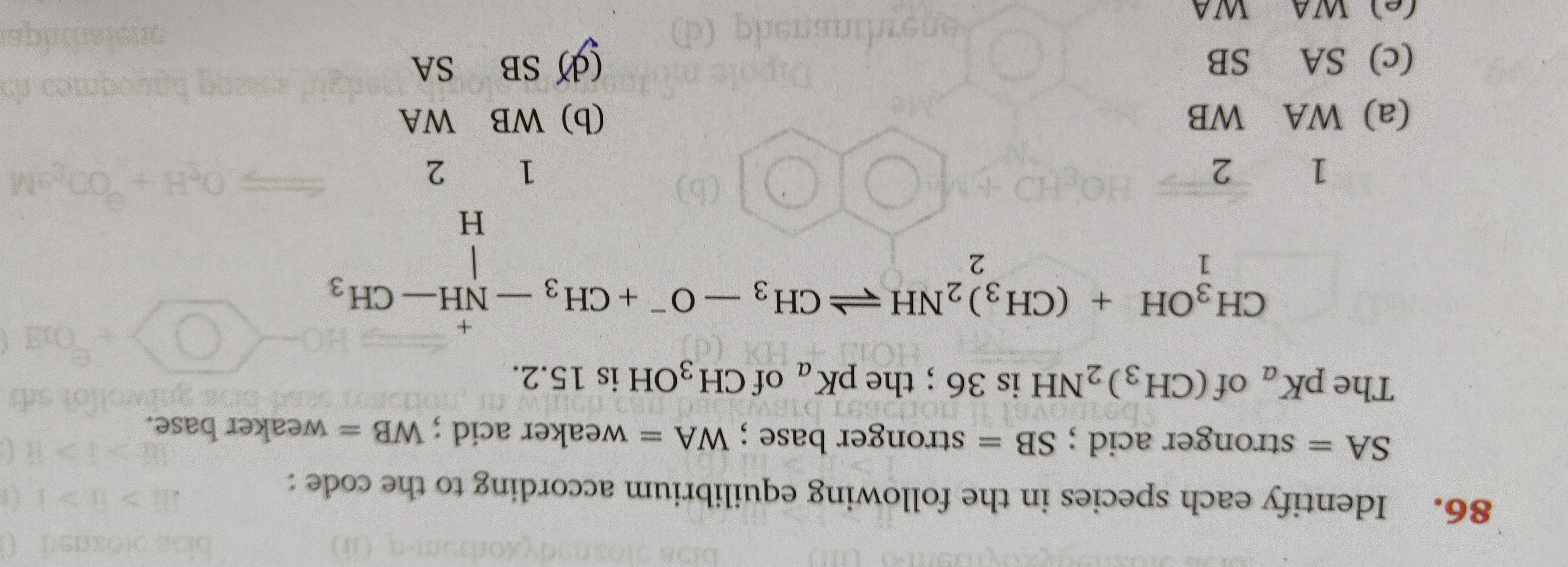
WA WB
WB WA
SA SB
SB SA
WA WA
SB SA
Solution
The pKₐ of CH₃OH is 15.2, making it a weaker acid (WA). Its conjugate base, CH₃O⁻, is therefore a stronger base (SB). The pKₐ of the conjugate acid of (CH₃)₂NH, which is (CH₃)₂NH₂⁺, is 36. This indicates that (CH₃)₂NH₂⁺ is a weaker acid (WA). Consequently, its conjugate base, (CH₃)₂NH, is a stronger base (SB).
The equilibrium is: CH₃OH (WA) + (CH₃)₂NH (SB) <=> CH₃O⁻ (SB) + (CH₃)₂NH₂⁺ (WA)
The numbering indicates: Species 1: CH₃OH and CH₃O⁻ Species 2: (CH₃)₂NH and (CH₃)₂NH₂⁺
If the question asks for the classification of the reactants in order (Species 1, Species 2): CH₃OH (WA), (CH₃)₂NH (SB) -> (WA, SB)
If the question asks for the classification of the products in order (Species 1, Species 2): CH₃O⁻ (SB), (CH₃)₂NH₂⁺ (WA) -> (SB, WA)
Given the correct answer is (d) SB SA, there is an inconsistency with standard acid-base classifications and typical question formats. However, if we interpret the question as asking for the classification of the stronger base and stronger acid involved in the equilibrium, or possibly a misinterpretation of the species numbering, the provided answer suggests a specific, albeit unconventional, interpretation.
Assuming the question intends to classify the species in a way that leads to "SB SA": If Species 1 is SB and Species 2 is SA. This would imply CH₃OH (or its conjugate) is SB, and (CH₃)₂NH (or its conjugate) is SA. This does not align with the pKₐ values.
Let's reconsider the definitions and the given answer. The answer is (d) SB SA. This implies that the classification for species 1 is SB and for species 2 is SA. This is highly problematic as CH₃OH is a WA and (CH₃)₂NH is a SB. There might be a misunderstanding of what "Species 1" and "Species 2" refer to in the context of the options.
However, if we assume the question is asking for the classification of the acid and base in the forward reaction, ordered as (Base, Acid), then: Base = (CH₃)₂NH (SB) Acid = CH₃OH (WA) Resulting in (SB, WA). This is close to option (b) if it were SB WA.
Let's assume the question is asking for the classification of the stronger base and stronger acid involved in the reaction. Stronger Base: (CH₃)₂NH (SB) Stronger Acid: CH₃OH (WA) This gives (SB, WA).
Given the provided answer is (d) SB SA, and the standard interpretation yields (WA, SB) or (SB, WA), it suggests an error in the question, options, or the provided answer key. However, if forced to align with (d) SB SA, it implies a non-standard interpretation where "Species 1" is considered SB and "Species 2" is considered SA, which contradicts the derived acid-base strengths.
