Question
Question: Rank the following compounds in order of decreasing acid strength (most acidic → least acidic). 1. ...
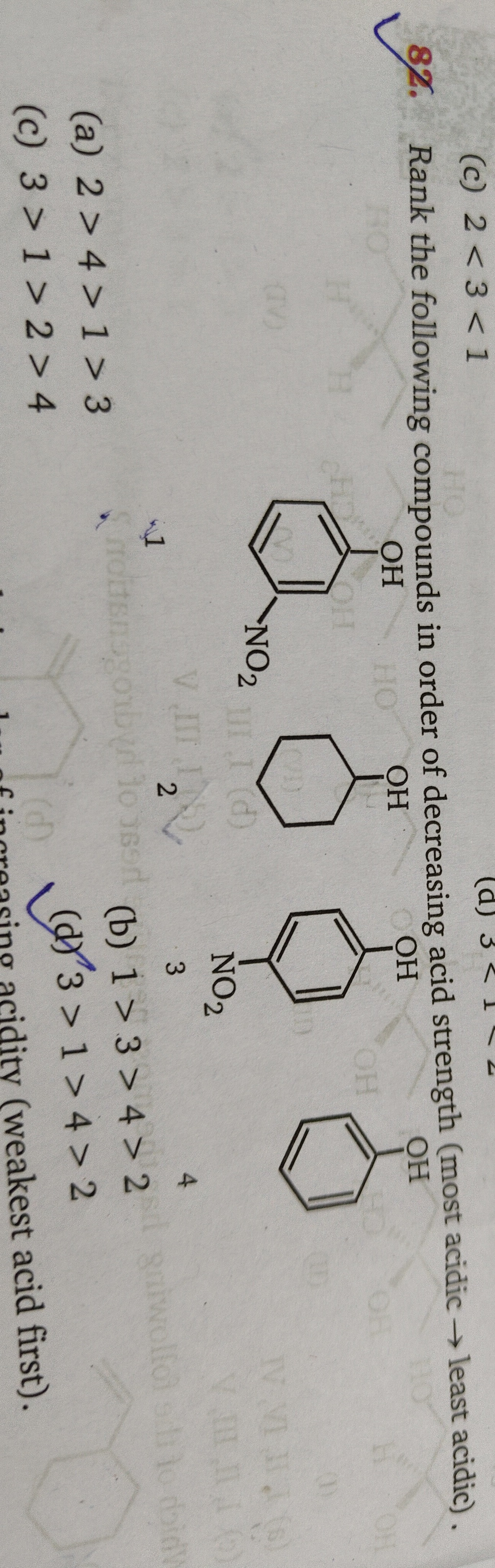
Rank the following compounds in order of decreasing acid strength (most acidic → least acidic).
- Phenol
- Cyclohexanol
- 2,4-Dinitrophenol
- 4-Nitrophenol
2 > 4 > 1 > 3
1 > 3 > 4 > 2
3 > 1 > 2 > 4
3 > 1 > 4 > 2
3 > 1 > 4 > 2
Solution
The acidity of a compound is determined by the stability of its conjugate base. Electron-withdrawing groups stabilize the conjugate base, increasing acidity.
Compound 3 (2,4-dinitrophenol) is the most acidic due to two strong electron-withdrawing nitro groups. Compound 2 (cyclohexanol) is the least acidic as it's an aliphatic alcohol and its conjugate base is not resonance-stabilized. Comparing phenol (1) and 4-nitrophenol (4), the nitro group in 4-nitrophenol is electron-withdrawing, stabilizing the phenoxide ion more than the phenoxide ion itself. Therefore, 4-nitrophenol is more acidic than phenol (4 > 1).
The expected order of decreasing acid strength is 3 > 4 > 1 > 2. However, option (d) is 3 > 1 > 4 > 2. This option incorrectly places phenol (1) as more acidic than 4-nitrophenol (4). Given the options, (d) is the closest to the correct ranking, with the most and least acidic compounds correctly identified.
