Question
Question: For the equilibrium $2SO_2(g) + O_2(g) \rightleftharpoons 2SO_3(g)$, $\Delta H = -198$ kJ, the equil...
For the equilibrium 2SO2(g)+O2(g)⇌2SO3(g), ΔH=−198 kJ, the equilibrium concentration of SO3 will be affected by
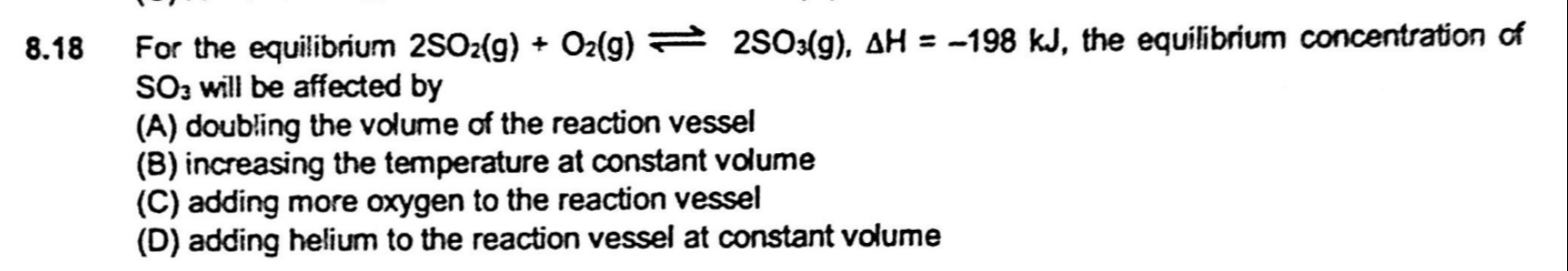
doubling the volume of the reaction vessel
increasing the temperature at constant volume
adding more oxygen to the reaction vessel
adding helium to the reaction vessel at constant volume
A, B, C
Solution
The problem asks how the equilibrium concentration of SO3 is affected by various changes for the given reaction:
2SO2(g)+O2(g)⇌2SO3(g)
ΔH=−198 kJ (This indicates the forward reaction is exothermic, releasing heat).
We will analyze each option based on Le Chatelier's Principle:
Le Chatelier's Principle: When a system at equilibrium is subjected to a change in concentration, temperature, pressure, or volume, the system will adjust itself to counteract the change and re-establish a new equilibrium.
Let's examine each option:
(A) Doubling the volume of the reaction vessel:
- Doubling the volume of the reaction vessel at constant temperature is equivalent to decreasing the total pressure of the system.
- According to Le Chatelier's Principle, if the pressure is decreased, the equilibrium will shift to the side with a greater number of moles of gas.
- In the given reaction:
- Moles of gaseous reactants (2SO2+O2) = 2 + 1 = 3 moles
- Moles of gaseous products (2SO3) = 2 moles
- Since the reactant side has more moles of gas (3) compared to the product side (2), decreasing the pressure (by doubling the volume) will shift the equilibrium to the left (reactant side).
- This shift will lead to a decrease in the equilibrium concentration of SO3.
- Therefore, this option affects the equilibrium concentration of SO3.
(B) Increasing the temperature at constant volume:
- The reaction is exothermic (ΔH=−198 kJ), meaning the forward reaction releases heat. We can write it as: 2SO2(g)+O2(g)⇌2SO3(g)+Heat
- According to Le Chatelier's Principle, if the temperature is increased, the equilibrium will shift in the direction that absorbs heat to counteract the added heat.
- Since the forward reaction is exothermic (releases heat), the reverse reaction is endothermic (absorbs heat).
- Increasing the temperature will shift the equilibrium to the left (reverse direction) to absorb the excess heat.
- This shift will lead to a decrease in the equilibrium concentration of SO3.
- Therefore, this option affects the equilibrium concentration of SO3.
(C) Adding more oxygen to the reaction vessel:
- Oxygen (O2) is a reactant in the given equilibrium.
- According to Le Chatelier's Principle, adding a reactant to the system at equilibrium will cause the equilibrium to shift in the direction that consumes the added reactant.
- Therefore, adding more oxygen will shift the equilibrium to the right (forward direction) to consume the added O2 and produce more SO3.
- This shift will lead to an increase in the equilibrium concentration of SO3.
- Therefore, this option affects the equilibrium concentration of SO3.
(D) Adding helium to the reaction vessel at constant volume:
- Helium (He) is an inert gas, meaning it does not participate in the chemical reaction.
- When an inert gas is added to a reaction vessel at constant volume, it increases the total pressure of the system. However, it does not change the partial pressures of the reacting gases (SO2, O2, SO3).
- Since the equilibrium constant (Kp) depends on the partial pressures of the reacting gases, and these partial pressures remain unchanged, the equilibrium position is not disturbed.
- Therefore, adding helium at constant volume will not affect the equilibrium concentration of SO3.
Based on the analysis, options (A), (B), and (C) all cause a change in the equilibrium concentration of SO3.
