Question
Question: The **CORRECT** statements about the structures of $H_2O_2, O_2F_2$ and $OF_2$ is/are:...
The CORRECT statements about the structures of H2O2,O2F2 and OF2 is/are:
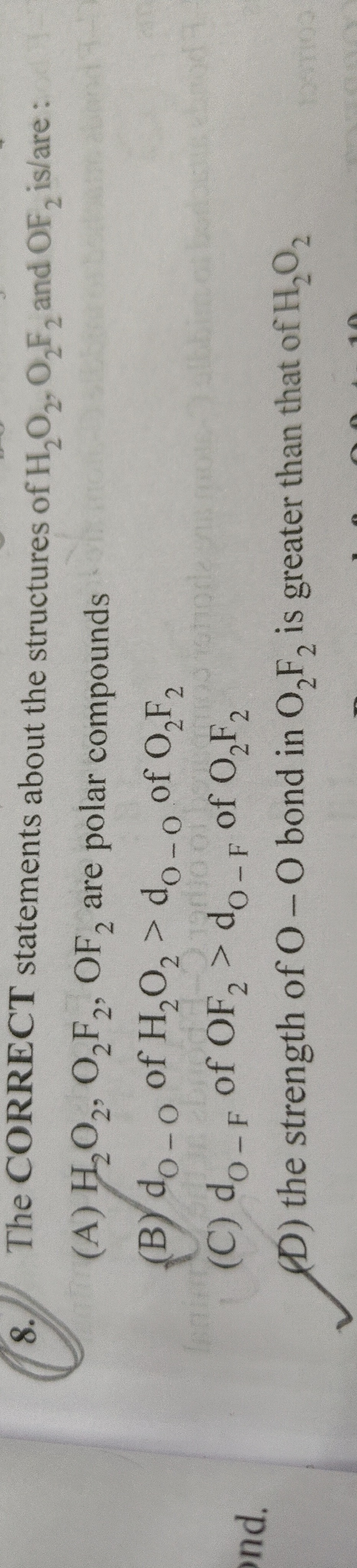
A
H2O2,O2F2,OF2 are polar compounds
B
dO−O of H2O2>dO−O of O2F2
C
dO−F of OF2>dO−F of O2F2
D
the strength of O−O bond in O2F2 is greater than that of H2O2
Answer
A, B, D
Explanation
Solution
The solution involves analyzing the structure, polarity, bond lengths, and bond strengths of the given compounds.
1. Analyze the polarity of H2O2,O2F2,OF2 (Statement A):
- H2O2 (Hydrogen Peroxide): Has a non-planar "open book" structure (H-O-O-H). The O-H bonds are polar, and due to the non-planar geometry, the bond dipoles do not cancel out, resulting in a net dipole moment. Thus, H2O2 is polar.
- O2F2 (Dioxygen Difluoride): Similar to H2O2, it has a non-planar "open book" structure (F-O-O-F). The O-F bonds are polar (Fluorine is more electronegative than Oxygen). Due to the non-planar geometry, the bond dipoles do not cancel out, resulting in a net dipole moment. Thus, O2F2 is polar.
- OF2 (Oxygen Difluoride): Has a bent (V-shaped) structure, similar to water. The central oxygen atom has two lone pairs and forms two polar O-F bonds. The bond dipoles do not cancel out due to the bent geometry, resulting in a net dipole moment. Thus, OF2 is polar.
- Conclusion for (A): All three compounds (H2O2,O2F2,OF2) are polar. Statement (A) is CORRECT.
2. Compare dO−O of H2O2 and O2F2 (Statement B):
- H2O2: The O-O bond is a single bond. The experimental O-O bond length is approximately 1.48 Å.
- O2F2: The O-O bond is also formally a single bond. However, due to the high electronegativity of fluorine, electron density is strongly withdrawn from the oxygen atoms. This reduces the lone pair-lone pair repulsion between the oxygen atoms and increases the effective nuclear charge, leading to an unusually short and strong O-O bond. The experimental O-O bond length is approximately 1.22 Å, which is even shorter than the O=O double bond in O2 (1.21 Å).
- Comparison: 1.48 A˚(H2O2)>1.22 A˚(O2F2).
- Conclusion for (B): Statement (B) is CORRECT.
3. Compare dO−F of OF2 and O2F2 (Statement C):
- OF2: The O-F bond in OF2 is a single bond. The experimental O-F bond length is approximately 1.41 Å.
- O2F2: The O-F bond in O2F2 is also a single bond. The experimental O-F bond length is approximately 1.58 Å.
- Comparison: 1.41 A˚(OF2)<1.58 A˚(O2F2).
- Therefore, dO−F of OF2 is not greater than dO−F of O2F2.
- Conclusion for (C): Statement (C) is INCORRECT.
4. Compare the strength of O−O bond in O2F2 and H2O2 (Statement D):
- Bond strength is inversely related to bond length. A shorter bond is generally stronger.
- From the analysis in (B), we found that dO−O of O2F2 (1.22 Å) is significantly shorter than dO−O of H2O2 (1.48 Å).
- Since the O-O bond in O2F2 is much shorter, it indicates that it is stronger than the O-O bond in H2O2.
- Conclusion for (D): Statement (D) is CORRECT.
Final Answer: Statements (A), (B), and (D) are correct.
