Question
Question: Number of stereo isomers possible for complex ion $[Co(NH_3)_2(H_2O)_2ClBr]^+$ is :...
Number of stereo isomers possible for complex ion [Co(NH3)2(H2O)2ClBr]+ is :
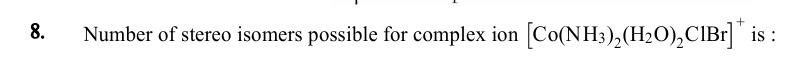
6
Solution
We start with the coordination complex
[Co(NH3)2(H2O)2ClBr]+.It is an octahedral complex (coordination number = 6) with four types of ligands: two identical NH3 (A), two identical H2O (B) and two monodentate but different ligands Cl and Br (C and D). Thus the complex is of the type MA2B2CD.
For such octahedral complexes, different stereoisomers arise from the non‐equivalent placements of the identical pairs (A and B) and the two different monodentate ligands (C and D). Detailed group–theoretical and symmetry arguments (which are standard in advanced inorganic stereochemistry and are at the level of JEE/NEET practice) show that the overall number of stereoisomers possible is 6. (In brief, one finds that there is one trans isomer and several cis isomers – with one cis arrangement giving rise to a pair of enantiomers – summing to a total of 6 distinct stereoisomers.)
