Question
Question: In each of the following pairs which will have higher enol content : (a) Diethyl malonate and Ethyl...
In each of the following pairs which will have higher enol content :
(a) Diethyl malonate and Ethyl acetoacetate
(b) Diethyl malonate and Methyl acetoacetate
(c) Pentan-3-one and Butan-2-one
(d) Dibenzyl ketone and Benzyl ethyl ketone
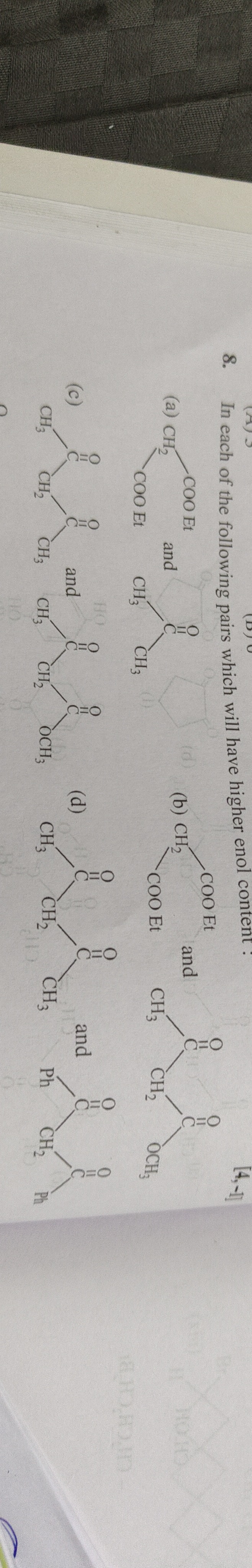
(a) Diethyl malonate, (b) Diethyl malonate, (c) Pentan-3-one, (d) Dibenzyl ketone
(a) Ethyl acetoacetate, (b) Methyl acetoacetate, (c) Butan-2-one, (d) Dibenzyl ketone
(a) Ethyl acetoacetate, (b) Diethyl malonate, (c) Butan-2-one, (d) Benzyl ethyl ketone
(a) Diethyl malonate, (b) Methyl acetoacetate, (c) Pentan-3-one, (d) Benzyl ethyl ketone
(a) Ethyl acetoacetate, (b) Methyl acetoacetate, (c) Butan-2-one, (d) Dibenzyl ketone
Solution
Enol content is favored by factors stabilizing the enol form, such as conjugation, aromaticity, and intramolecular hydrogen bonding. β-keto esters (like ethyl acetoacetate and methyl acetoacetate) show significant enol content due to resonance and H-bonding. Compounds with phenyl rings conjugated to the enol double bond (dibenzyl ketone, benzyl ethyl ketone) also show increased enol content, with dibenzyl ketone being more stabilized. For simple ketones, the stability of the enol double bond (more substituted, more hyperconjugation) influences the enol content.
