Question
Question: How many of the following species, the negative charge is delocalised? ...
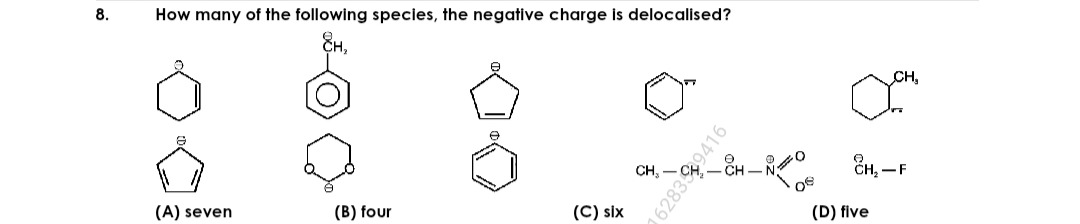
How many of the following species, the negative charge is delocalised?
A
seven
B
four
C
six
D
five
Answer
B
Explanation
Solution
To determine if a negative charge is delocalized, we look for the possibility of resonance. Resonance occurs when a lone pair of electrons (forming the negative charge) is adjacent to a pi bond (double or triple bond), a vacant p-orbital, or another atom with a lone pair that can participate in conjugation.
After analyzing each species, the species with delocalized negative charges are:
- Benzyl anion
- Cyclopenta-2,4-dien-1-ide anion
- Cyclohex-2-en-1-ide anion
- 2-Nitropropan-2-ide anion
There are a total of four such species.
